REVIEW ARTICLE
Novel Therapeutic Strategies Targeting Molecular Pathways of Cystogenesis in Autosomal Polycystic Kidney Disease
Maurizio Salvadori1, Aris Tsalouchos2
1Renal Unit, Careggi University Hospital, Viale Pieraccini, Florence, Italy; 2Division of Nephrology, Azienda Ospedaliera Careggi, Largo Alessandro Brambilla, Florence, Italy
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease that results from mutations in PKD1 or PKD2. The disease is characterized by the progressive development of fluid-filled cysts derived from renal tubular epithelial cells that destroy the architecture of the renal parenchyma and lead to kidney failure. Until recently, the causes and the molecular pathways that lead to cystogenesis remained obscure. In the last decade, enormous progress has been made in understanding the pathogenesis of ADPKD and the development of new therapies. The purpose of this review is to update on the promising therapies that are being developed and tested based on knowledge of recent advances in molecular and cellular targets involved in cystogenesis.
Keywords: adult autosomal polycystic kidney disease; cystogenesis; mTOR signaling; somatostatin analogues; vasopressin 2 receptors
Received: 31 January 2017;
Accepted after revision: 16 February 2017;
Published: 15 March 2017.
Author for correspondence: Maurizio Salvadori, Renal Unit, Careggi University Hospital, Viale Pieraccini, 18, 50139, Florence, Italy. Email:
maurizio.salvadori1@gmail.comHow to cite: Salvadori M et al. Novel Therapeutic Strategies Targeting Molecular Pathways of Cystogenesis in Autosomal Polycystic Kidney Disease. J Ren Hepat Disord 2017;1(1):35–49.
DOI:
http://dx.doi.org/10.15586/jrenhep.2017.10Copyright: Salvadori M and Tsalouchos A.
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) affects 1:400-1:1.000 live births, or 12.5 million people worldwide, and is the most common monogenic inherited form of kidney disease across all ethnic types. ADPKD is characterized by cyst formation and enlargement in the kidney and other organs. It accounts for 5% to 10% of end-stage renal disease (ESRD) cases, making it the fourth leading global cause for kidney failure with clinically significant impairment of renal function. ESRD usually occurs by late middle age and requires renal replacement therapy in approximately 50% of patients by 70 years of age (1–2).
In 85% of cases, ADPKD occurs as a result of germline mutation in polycystin 1 gene (PKD1) localized within chromosome 16 [16p13.3], while in 15% of cases, it is due to germline mutation in polycystin 2 gene (PKD2) localized within chromosome 4 [4q21-q23] (3). Although some authors have supposed that there is a third PKD gene, convincing evidence to support the existence of this gene is lacking. Polycystin-1 (PC1) and polycystin-2 (PC2) interact with each other through their C-terminal cytoplasmic domains and are known to form a complex that functions as a transient receptor potential channel involved in the regulation of intracellular calcium homeostasis (4,5). On average, patients with mutations in PKD1 develop ESRD at younger ages compared with patients (aged 54.3 vs. 74 years) with PKD2 mutations (6).
According to a widely accepted view, cystogenesis follows a two-hit model. ADPKD is recessive at the cellular level and cysts develop clonally from a tubular cell only once the cell has acquired a second somatic mutation to inactivate the remaining normal allele (7). A somatic “second-hit” mutation, loss of heterozygosity, or haploinsufficiency may account for the mosaic nature of cyst formation, while in the mature organ, broad and fast cyst formation requires a third hit such as kidney injury (8–10). Recent evidences suggest that a complete loss of function is not required for cystogenesis; rather, functional PC1 or PC2 must be reduced to a certain threshold level (1).
Although the exact mechanisms of cystogenesis remain to be elucidated, the pathological processes that facilitate cyst enlargement are probably the result of two specific abnormalities: (1) increased fluid secretion into the cyst lumen and (2) inappropriately increased cell division by the epithelium lining the cyst (11). The major signaling pathways implicated in these phenotypic changes include: the intracellular deregulation of calcium homeostasis, cAMP accumulation and activation of protein kinase A (PKA), activation of mitogen-activated protein and mammalian target of rapamycin (mTOR) kinases, canonical Wnt signaling, and other intracellular signaling mechanisms (12–14).
Until recently, treatment of ADPKD was aimed at the management of secondary conditions, particularly hypertension, to limit morbidity and mortality, after which the disease becomes symptomatic. The recent developments arising from a better mechanistic understanding of the molecular pathways involved in cyst growth have allowed for targeting the disease pathogenesis, rather than the disease complications. In this context, novel therapies with strong molecular rationales have entered into clinical trials as potentially modifying ADPKD. A significant factor propelling these trials is the now accepted total kidney volume (TKV) imaging technology by magnetic resonance imaging (MRI). According to the findings of the Consortium of Radiologic Imaging Studies of PKD (CRISP), TKV correlates well with dimensions of cysts within the kidneys and with eGFR. The rate of renal growth is a good predictor of renal functional decline, and thus it can be used as a surrogate marker of disease progression in clinical trials, along with measured glomerular filtration rate (GFR) or serum creatinine change as principal meaningful end points (15,16). The current review focuses on these novel therapeutic approaches that interfere with the molecular pathways of cystogenesis (Figure 1).
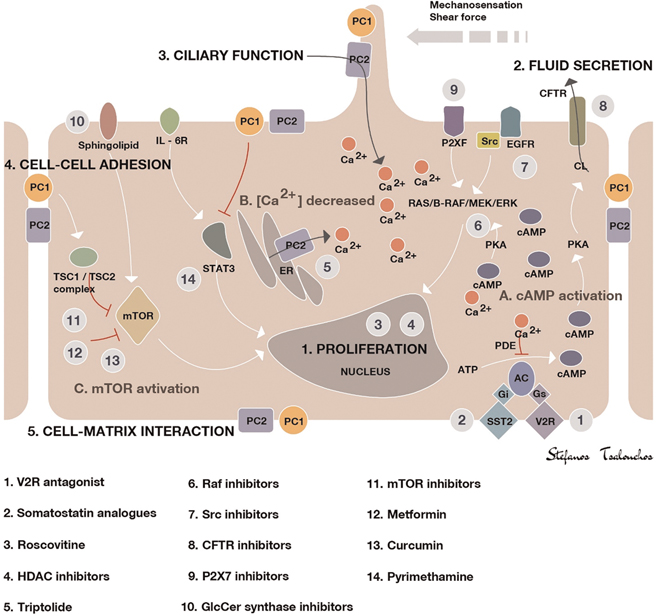
Figure 1. Illustration of the key mechanisms of ADPKD pathogenesis and targets of potential treatments. Polycystin-1 and polycystin-2 expressed in different subcellular locations and regulate (1) proliferation, (2) fluid secretion, (3) ciliary function, (4) cell–cell adhesion, and (5) cell–matrix interaction of renal epithelial cells. Dysfunction of polycystin-1 or polycystin-2 results in aberrant signaling pathways, including: (A) activation of cAMP, (B) decreased intracellular calcium concentrations, and (C) activation of mTOR. The targets of candidate drugs are depicted as gray circles. Abbreviations: CFTR, cystic fibrosis transmembrane regulator; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; GlcCer, glucosylceramide; HDAC, histone deacetylase; IL-6R, interleukin-6 receptor; MEK, mitogen-activated protein kinase; mTOR, the mammalian target of rapamycin; PC, polycystin; PDE, phosphodiesterase; PKA, protein kinase A; SR, somatostatin receptor; TSC, tuberous sclerosis; V2R, vasopressin V2 receptor.
Drugs Targeting cAMP-Dependent Cystic Expansion
Role of cAMP in cystogenesis
In ADPKD, disruption of intracellular Ca2+ homeostasis due to mutations in the PKD genes leads to low intracellular calcium and consequently increased levels of intracellular cAMP. Normally, the levels of cAMP are controlled by a balanced activity of membrane-bound (under the control of G protein–coupled receptors (GPCRs] and extracellular ligands) and soluble isoforms of adenylate cyclase (AC), which catalyzes the formation of cAMP from ATP, and phosphodiesterases (PDEs), which degrades cAMP to AMP. Decreased intracellular calcium inhibits the activity of PDEs and activates ACs, thus producing a net increase in cAMP concentration (12). cAMP exerts its effects via PKA which phosphorylates a number of metabolic enzymes and promotes transepithelial fluid secretion, which involves chloride secretion through the cystic fibrosis transmembrane conductance regulator (CFTR). Chloride secretion drives sodium into the cystic cavity through paracellular mechanisms; this causes movement of water through aquaporins and cyst’s expansion (17,18). In addition, despite the evidence suggesting that cAMP is antimitogenic in normal cells, in ADPKD cAMP promotes cyst enlargement by stimulating epithelial cell proliferation through the activation of the B-Raf/MEK/ERK pathway (19–22). cAMP/PKA signaling is also involved in cell proliferation by activating mTOR (via ERK-mediated phosphorylation of tuberin) (23,24) and Wnt–β-catenin signaling (via phosphorylation of GSK3b and β-catenin) (25,26). In addition, cAMP upregulates the paired box gene 2 (Pax2) (27) and signal transducer and activator of transcription 3 (STAT3) (28–30).
Vasopressin 2 receptor antagonists
Normally, vasopressin (AVP) is secreted into the circulation by the posterior pituitary gland, in response to an increase in serum osmolality or a decrease in effective circulating volume. In the kidney, AVP binds to the V2 vasopressin receptor in the basolateral membranes of collecting duct cells in the last portion of the nephron. The V2 receptor is a typical member of the large superfamily of GPCRs. Thus, occupancy of this receptor results in mediated activation of AC and the formation of cAMP with a subsequent activation of PKA, which promotes the fusion of cytoplasmic vesicles containing aquaporin-2 water-channel proteins with the apical membrane. As a result, this normally water-tight membrane becomes water-permeable. Driven by the osmotic gradient of sodium, water is then transcellularly reabsorbed, entering the cells through aquaporin-2 in the apical membrane and leaving the cells for the interstitium through aquaporin-3 and aquaporin-4, which reside in the basolateral membrane (31). In patients with ADPKD, there is a pathologically hyperactive AVP/V2 receptor system. The altered regulation of AVP serum levels in ADPKD have been reanalyzed in a number of recent studies. Serum concentrations of vasopressin correlate positively with both serum osmolality as well with total kidney size and correlates negatively with GFR in ADPKD patients (32–35). There are two major reasons for the deregulation of the neurohypophysis–kidney axis in ADPKD. First, changes in kidney architecture cause a urinary concentration deficit with subsequently elevated plasma osmolalities. Second, an altered central release of vasopressin might exist. Indeed, Ahrabi et al. (36) observed a syndrome of inappropriate antidiuretic hormone secretion-like phenotype in PKD1 haploinsufficient rodents. These rodents were able to reabsorb abnormally high amounts of water supporting the idea of a nontubular basis of the urinary concentration deficit. In addition, Ho et al. (37) by investigating the osmoregulation parameters in adult and pediatric ADPKD patients with intact GFR in comparison with nonaffected controls, showed a significant defect both in the release of vasopressin in response to plasma osmolality (central component) and in the V2R-mediated response (nephrogenic component) in ADPKD patients. Because of the aforementioned central role of cAMP in cystogenesis and the pathologically hyperactive AVP/V2 receptor system in ADPKD patients, the block of the effect of AVP on V2R is particularly appealing as AVP is the major hormone responsible for cAMP generation in isolated collecting ducts (38). Nagao et al. (39) documented the importance of circulating AVP in the natural progression of the disease by suppression of AVP with high water intake in PCK rats. The authors observed that an increased water intake, for 10 weeks, reduced renal cell proliferation, cystic area, and kidney weight, and improved renal function.
In preclinical trials, a nonpeptide vasopressin antagonist mozavaptan (OPC-31260), administered in murine cystic models orthologous to human disease, including the Pkd2WS25/− mouse (ADPKD), PCK rat (ARPKD), and pcy mouse (nephronophthisis type 3), reduced renal cAMP and inhibited disease progression measured by the reduction in kidney volume, the cystic area, the number of mitotic and apoptotic cells, and the blood urea nitrogen (BUN) (40–42). However, initiation of mozavaptan in Pkd1-deletion mouse model later in the disease progression did not reduce cyst formation, suggesting that early initiation of V2 receptor antagonism is most effective to decrease disease progression (43). Additional studies were conducted to examine the effects of tolvaptan (OPC-4106), a more potent and highly selective human V2R antagonist, in comparison with mozavaptan (44). Tolvaptan showed similar results on renal cAMP and PKD progression in PCK rat model using the lowest dose, which caused only modest aquaresis compared with the higher dose regimens. There was also a corresponding reduction in the renal activity of the B-Raf/MEK/ERK pathway (45). Subsequently, Reif et al. (46), in an in vitro study, examined the effect of tolvaptan on intracellular cAMP, ERK activity, cell proliferation, and transcellular chloride anion secretion using human ADPKD cyst epithelial cells. Tolvaptan caused inhibition of cAMP AVP–induced production, ERK signaling AVP–induced, cell proliferation, and chloride anion secretion. These effects significantly contributed to a decrease in in vitro cyst growth. Wang et al. (47) confirmed that the effect of these drugs in reducing disease progression and cyst development was due to the inhibition of AVP effects by selectively knocking out AVP in the PCK rat. In the absence of AVP, the PCK rat had reduced renal cAMP accumulation, ERK activity, cell proliferation, and fibrosis, and was essentially free of renal cysts, whereas administration of the V2R agonist 1-deamino-8- d-arginine vasopressin fully rescues the cystic phenotype, providing clear evidence for the roles of AVP and cAMP on cystic disease progression.
The large, randomized, double-blind, placebo-controlled, multinational, phase III TEMPO 3:4 trial (48) confirmed the aforementioned experimental studies. This trial enrolled 1445 patients aged 18–50 years with ADPKD, rapidly progressive kidney growth (TKV ≥ 750 mL), as measured by MRI and CKD stages 1–3. Tolvaptan reduced the rate of TKV growth (primary endpoint) by 49% and the rate of estimated GFR (eGFR) loss on treatment (secondary endpoint) by 26% per year during the median observation period of 3 years. The effect on TKV appeared greater during the first year of treatment than during the second or third years. In addition, the beneficial effects on renal function observed in all patient subgroups were greater in patients aged ≥35 years and in patients with hypertension or a TKV of ≥1500 mL. An early and reversible small reduction in GFR at the start of tolvaptan therapy, observed also in previous short-term trials (49–51), was likely caused by alterations in tubuloglomerular feedback and/or renal hemodynamics. Another important secondary endpoint was the reduction in kidney pain occurring early and throughout treatment. The results of TEMPO 3:4 trial suggested that tolvaptan had no effect compared with placebo on albuminuria. Conversely, a post hoc exploratory analysis documented that tolvaptan decreased albuminuria compared with placebo, also independently of blood pressure. In addition, the treatment efficacy of tolvaptan on changes in TKV and eGFR was more readily detected in patients with higher albuminuria (52). The drug was assumed in a split dose regimen of 45 mg in the morning and 15 mg in the afternoon, uptitrated to 90/30 mg when tolerated. Split dose regimen was preferred based on the findings of phase II studies (TEMPO 2:4 and 156-05-002) in patients with ADPKD that documented that a split dose regimen was more effective than a single dose in achieving sustained vasopressin suppression, as evidenced by a 24-h urine osmolality (53,54).
Based on the results of the TEMPO 3:4 trial, tolvaptan has been approved to delay the progression of ADPKD in patients with a rapid increase in TKV in Japan, Canada, European Union, United Kingdom, and South Korea. However, eligibility criteria for the prescription vary between countries (Table 1). The European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) (55) and the Renal Association Working Group on Tolvaptan in ADPKD (56) have issued more detailed guidance on this topic.
Table 1. Eligibility criteria for the approved use of tolvaptan according to country or region
| Country |
Chronic kidney disease stage |
Disease activity |
Regulatory body |
Approval date |
Guidance (if any) |
| Japan |
1–4 |
TKV > 750 mL
ΔTKV > 5% per annum |
Pharmaceuticals and Medical Devices Agency |
March 2014 |
|
| Canada |
Not specified |
Not specified |
Health Canada |
February 2015 |
|
| Europe |
1–3 |
Evidence of rapid disease progression |
European Medicines Agency |
May 2015 |
European Renal Association-European Dialysis and Transplant Association |
| England, Wales, and Northern Ireland |
2–3 |
Evidence of rapid disease progression |
National Institute for Health and Clinical Excellence (NICE) |
October 2015 |
Renal Association |
| South Korea |
1–3 |
Evidence of rapid disease progression |
Ministry of Food and Drug Safety/Health Insurance Review and Assessment Service |
December 2015 |
|
| Scotland |
1–3 |
Evidence of rapid disease progression |
Scottish Medicines Consortium |
January 2016 |
Renal Association |
| TKV, total kidney volume. |
Although the results of the TEMPO trial are highly encouraging, tolvaptan cannot be considered as an option for all patients. Tolvaptan has significant adverse effects, including aquaretic effects (polyuria, nocturia, polydipsia) and elevation of aminotransferase enzyme concentrations with the potential for acute liver failure (48,57,58). Appropriate patient selection is critical to optimize long-term benefits while minimizing adverse effects and hepatotoxic risk factors.
Studies to further assess the efficacy and tolerability of tolvaptan in patients with ADPKD are ongoing or just completed. TEMPO 4:4 is a 2-year, open-label extension of TEMPO 3:4, completed in March 2016. This study aimed to evaluate the long-term efficacy and safety of tolvaptan in patients with ADPKD; the findings are waiting to be published soon. In addition, the long-term safety of titrated tolvaptan in patients with ADPKD is currently being assessed in a phase III open-label trial (NCT02251275) (59), while the ongoing phase IIIb REPRISE trial aims to extend the understanding of the efficacy and safety of tolvaptan in patients with late stage 2 to early stage 4 CKD (60).
Somatostatin analogues
Somatostatin (SST) is an endogenous hormone secreted by the pancreatic islet δ-cells and by extra-islet neuroendocrine cells of the gastrointestinal tract, hypothalamus, and thyroid. SST has anti-secretory and anti-proliferative effects mediated by the interaction with five subtypes of GPCRs (SSTR1-5) (61). SST receptors are expressed by renal tubular epithelial cells and by cholangiocytes. In particular, SSTR1 and SSTR2 are expressed in the ascending limb of Henle’s loop, the distal tubule and collecting duct, while SSTR3, SSTR4 and SSTR5 are expressed in the proximal tubule (62–65). It has been shown that SST selectively inhibits cAMP synthesis in the epithelial cells of the distal tubules and collecting ducts both in vitro and in vivo (66,67) and exerts similar effects to cholangiocytes (68). As plasma half-life of the native SST is very short (1–3 min), the synthetic analogues octreotide, lanreotide, and pasireotide were developed as stable alternatives for use in clinical practice. These analogues of SST differ in their stability and affinity with the SST receptors.
In particular, octreotide and lanreotide have a half-life of 2 hours and present a high affinity for SSTR2 and SSTR3 and moderate affinity for SST5. Instead, pasireotide has high affinity for all the receptors of SST, except SSTR4, and its plasma half-life is about 12 hours (69). Currently, formulations of octreotide and lanreotide with long acting release (LAR), which allow their administration every 28 days intramuscularly or intradermally, have been introduced into clinical practice. Ruggenenti et al. (70) have evaluated for the first time the effectiveness of octreotide-LAR by performing a randomized, cross-over, placebo-controlled trial in 14 ADPKD patients, which demonstrated the potential efficacy in slowing the growth of TKV and the relative safety of the treatment. A post hoc analysis of the same study showed that the volume of the liver cysts decreased significantly with octreotide-LAR (71). Following this initial experience, a number of preclinical studies have confirmed the effectiveness of treatment with SST analogues in inhibiting the growth of renal and hepatic cysts. Bogert et al. (72) developed a zebrafish model that allows for the testing of the possible efficacy of drugs in inhibiting hepatorenal cystogenesis. In this experimental model, the exposure of zebrafish embryos to pasireotide significantly reduced the area of the cysts. A recent study also assessed the efficacy of pasireotide and tolvaptan, in a murine model of ADPKD (73). Treatment with pasireotide or tolvaptan alone, significantly reduced the growth of cysts, and the effect was even more marked by combining the two drugs. In another randomized study, Van Keimpema et al. (74) compared the effects of 6 months of treatment with lanreotide or placebo in 54 patients with polycystic liver disease (PLD), including 32 with ADPKD and the remaining with isolated polycystic liver disease (PCLD). The average volume of the liver decreased in patients treated with lanreotide while it increased in the placebo group. Moreover, in patients with ADPKD, TKV was reduced after treatment with lanreotide while it increased in the placebo group. In a subsequent open-label extension study (75), patients who participated in the initial trial were re-enrolled to complete a treatment period of 12 months with lanreotide. Liver volume decreased after 12 months of treatment with lanreotide, with the greatest effect seen during the first 6 months. In the 25 patients with ADPKD, TKV remained stable at the end of 12 months. Furthermore, in 15 patients with ADPKD, a CT of the kidneys was repeated 6 months after the treatment with evidence of increased TKV during this period. In another 12 months of study, 42 patients with PLD, including 34 with ADPKD, were randomized to receive treatment with octreotide-LAR or placebo (76). The total volume of the liver was reduced in the treatment arm with octreotide-LAR but increased in the placebo group. In patients with ADPKD, the TKV remained unchanged in the octreotide-LAR group but increased in the placebo group. In addition, renal function had a slower reduction in patients treated with octreotide-LAR, although the difference did not reach statistical significance. This study also had an open-label extension for 12 months (77). In the group initially randomized to octreotide-LAR, the reduction of liver volume remained evident until the end of the second year of treatment, although the effect was not significant during the second year. Instead, in the originally randomized placebo group who continued with octreotide-LAR, the total volume of the liver decreased significantly after 1 year of treatment with the drug. In the cohort of patients with ADPKD initially randomized to octreotide-LAR, the inhibition of kidney growth observed during the first year was not observed in the second year of study, while in those originally randomized to placebo, TKV remained unchanged after 1 year of treatment with octreotide-LAR. More recently, in the ALADIN multicenter study conducted in Italy, 79 patients with ADPKD and eGFR > 40 mL/min/1.73 m2 were randomized to a 3-year treatment with octreotide-LAR or placebo (78). After the first year, the average increase in TKV was significantly lower in patients treated with octreotide-LAR compared with those receiving placebo. In the third year, the average increase in TKV in the treatment arm was lower than the placebo group without reaching statistical significance. During the entire study period, the annual reduction in GFR was lower in octreotide-LAR group than in the placebo group, although the difference did not reach statistical significance. However, a further analysis documented that while the reduction of GFR after 1 year was comparable in the two groups, the chronic loss of renal function between the first and third year was significantly slower in the treatment arm compared with placebo group, with a difference of about 50%. A more recent open-label clinical study evaluated the efficacy of 6 months of treatment with lanreotide in 43 patients with symptomatic PLD and ADPKD (estimated GFR > 30 mL/min/1.73 m2) (79). Compared with baseline, the median liver volume as well as that of the kidney decreased significantly. In addition, renal function remained stable until the end of the study. A recent meta-analysis confirmed the efficacy of SST analogues in reducing the progressive increase of TKV, on average with a reduction of 9% compared with the growth observed in patients treated with placebo or conventional therapies. However, treatment with SST analogues did not demonstrate significant effects on the eGFR (80). Based on these studies, in August 2015, European Medicines Agency (EMA) attributed to lanreotide the Orphan Drug designation for the treatment of ADPKD.
In the studies mentioned above, treatment with SST analogues was generally well tolerated with no particular problems, diarrhea being the most common adverse event. However, recently, the authors of a randomized, controlled clinical trial assessing the efficacy of lanreotide to halt disease progression in patients with later stage ADPKD (NCT01616927) documented an increased risk for hepatic cyst infection during lanreotide treatment, especially in ADPKD patients with a history of hepatic cyst infection. A literature review also suggested an increased risk for hepatic cyst infection during the use of somatostatin analogues (81).
Additional clinical trials of SST analogues for ADPKD and/or PLD are currently ongoing [NCT01616927: Study of Lanreotide to Treat Polycystic Kidney Disease (DIPAK1); NCT01377246: Somatostatin in Patients with Autosomal Dominant Polycystic Kidney Disease and Moderate to Severe Renal Insufficiency (ALADIN 2); NCT02127437: Lanreotide in Polycystic Kidney Disease Study (LIPS); NCT01670110: Pasireotide LAR in Severe Polycystic Liver Disease (SOM230)].
Drugs Targeting mTOR Signaling Pathway
Role of mTOR signaling pathway in cystogenesis
Serine/threonine-protein kinase mTOR is an enzyme that plays a critical role in proliferation and cell growth (82). The first suggestion of a prominent role of mTOR pathway in the pathogenesis of ADPKD comes from studies in patients with severe infantile-onset of ADPKD due to a large deletion of chromosome 16 involving PKD1 gene [16p13.3] and the adjacent tuberous sclerosis 2 (TSC2) gene [16p13.3] (83). Mutations in TSC1 [9q34.13] or TSC2 are the causes of tuberous sclerosis complex, an autosomal-dominant, neurocutaneous, multisystem disorder characterized by the formation of benign hamartomas in various organs including kidneys that are also involved with bilateral cysts formation (84). TSC1 and TSC2 encode, respectively, for hamartin and tuberin. These two proteins together with TBC1 domain family member 7 (TBC1D7) form the TSC protein complex that acts as a critical negative regulator of mTOR complex 1 (mTORC1) (85). PC1 also has an important function in the regulation of the mTOR pathway, as C-terminal cytoplasmic tail of PC1 interacts with tuberin. In ADPKD, this interaction is impaired and the mTOR pathway is inappropriately activated in cyst-lining epithelial cells of human ADPKD patients and mouse models (86). Based on these data, mTOR inhibitors have been suggested as possible therapeutic agents for ADPKD.
mTOR inhibitors
Sirolimus, a macrocyclic lactone produced by fermentation of Streptomyces hygroscopicus, exerts potent antiproliferative and antifibrotic effects by inhibiting the mTOR pathway. Sirolimus and its derivative everolimus, used in maintenance immunosuppression in patients undergoing kidney transplantation, have been proposed as potential new drugs to slow the growth of cysts and the progression of ADPKD in ESRD. The effects of treatment with mTOR inhibitors have been assessed in different experimental models of ADPKD (87–90). An early study showed that in Han:SPRD rats, the administration of sirolimus for 5 weeks significantly reduced the proliferation of tubular cells, inhibited cystogenesis and the growth of the kidney, and preserved renal function (91). More recently, prolonged treatment with sirolimus, in the same experimental model, has been observed to normalize the volume of the kidney, renal function, and blood pressure (92). These experimental studies have provided the rationale for the design of several prospective clinical trials aimed to verify the effectiveness of treatment with mTOR inhibitors in ADPKD patients. A first published randomized double-blind study, compared the effects of 2 years of treatment with everolimus (5 mg/day) or placebo in 433 patients with ADPKD and GFR > 30 mL/min/1.73 m2 (93). During the first year of study, the increase of TKV was significantly lower in the treatment arm with everolimus compared with placebo. This effect was not confirmed at the end of the second year. In addition, the initial effectiveness of everolimus in slowing down TKV did not translate into an improvement in renal function. An important bias of this study could be the high proportion of patients who discontinued the study in the everolimus group due to poor tolerability to treatment with the mTOR inhibitor. Indeed, the urinary protein excretion was significantly increased, and the overall incidence of adverse events was higher in the everolimus group; hyperlipidemia, leukopenia, thrombocytopenia, acne, stomatitis, and peripheral edema were the most relevant events. The SUISSE study compared the effects of treatment for 18 months with sirolimus (2 mg/day) or conventional therapy in 100 patients with ADPKD and GFR ≥ 70 mL/min/1.73 m2 (94). The median increase in TKV was comparable between the two groups as well as the eGFR throughout the entire study period. In this study, proteinuria was also significantly higher in patients treated with sirolimus than in the control group. The randomized trial SIRENA compared the effects of treatment with sirolimus or with conventional therapy alone for 6 months in 21 patients with ADPKD and GFR ≥ 40 mL/min/1.73 m2 (95). The treatment with sirolimus was associated with a minor increase of the TKV compared with conventional therapy, although the difference did not reach statistical significance. Even in this trial, appreciable variations of GFR were not observed, and as in the above described studies, the same adverse events (proteinuria, hyperlipidemia, thrombocytopenia, stomatitis) were presented. In a subsequent open-label study (RAPYD), 55 patients with ADPKD and mild to moderate renal impairment were randomized to 24 months of treatment with ramipril (control group), ramipril in combination with high doses of sirolimus (target blood levels: 6–8 ng/mL), or ramipril in combination with low-dose sirolimus (target blood levels: 2–4 ng/mL) (96). Compared with baseline, total cyst volume decreased significantly in both treatment arms with sirolimus, while it increased in the control group. Similarly, eGFR remained relatively stable in both treatment groups with sirolimus but worsened in the control group. However, at the end of 2 years of treatment, the urinary excretion of proteins and the incidence of hyperlipidemia were significantly higher in patients treated with high doses of sirolimus compared with the control group. In a more recent study, 30 patients with ADPKD and measured GFR ≥ 25 mL/min/1.73 m2 were randomized to receive for 12 months low-dose sirolimus (target blood levels 2–5 ng/mL), standard doses of sirolimus (target blood levels >5–8 ng/mL), or conventional therapy (97). TKV did not change significantly in the two treatment groups with sirolimus as in the group assigned to conventional therapy. In addition, the renal function improved (GFR measured by plasma clearance of iothalamate) with low-dose sirolimus, but not with the standard dose of the drug. Various hypotheses have been formulated to explain the discrepancies between the results obtained in experimental studies and in clinical trials. It is possible that the dose of sirolimus or everolimus used in clinical trials is not sufficient to inhibit mTOR enzyme activity in renal tubular cells or that the treatment was started in a too advanced stage of the disease to obtain an improvement of the renal function (86,87,98). In conclusion, the available clinical studies discourage the use of mTOR inhibitors to slow the progression of renal disease in patients with ADPKD. Currently there are two ongoing trials that test mTORs in ADPKD: Pulsed Oral Sirolimus in Autosomal Dominant Polycystic Kidney Disease (RAP) [NCT02055079] and The Efficacy of Everolimus in Reducing Total Native Kidney Volume in Polycystic Kidney Disease Transplanted Recipients (EVERKYSTE) [NCT02134899].
Other Therapeutic Targets in Preclinical Studies and Early Clinical Trials
Tyrosine kinase inhibitors
c-Src and Bcr-Abl are two cytoplasmatic tyrosine kinases (TKs) involved in the development of malignancies (99). Yamaguchi et al. (100) documented that in renal epithelia, the switch of cAMP from an anti-mitogen to a mitogenic stimulus not only correlates with decreased intracellular calcium levels but is also associated with an increased activity of Src. Furthermore, Sweeney et al. (21) evaluated bosutinib (SKI-606), a Src/Abl tyrosine kinase inhibitor, in the BPK and PCK rodent models of ADPKD. Bosutinib was found to suppress kidney cyst formation by inhibiting epidermal growth factor receptor (EGFR) activation and downregulating B-Raf/ERK signaling. In addition, bosutinib was effective in inhibiting epithelial cell proliferation and reducing extracellular matrix adhesion in an in vitro study on mouse inner medullary collecting duct cells and human ADPKD cyst-lining epithelial cells. In the same study, the authors also highlighted the ability of bosutinib to delay renal cystic phenotype of Pkd1 orthologous ADPKD heterozygous mice in vivo (101). Based on these evidences, a phase II, multicenter, randomized, double-blind, placebo-controlled clinical trial with bosutinib [NCT01233869] has been conducted and completed, and we currently are expecting publication of the study results. Tesevatinib, a new tyrosine kinase inhibitor, is being currently evaluated in two ongoing trials [NCT02616055 and NCT01559363].
Stimulation of polycystin-2-mediated Ca2+ release
Triptolide, a natural active component derived from the traditional Chinese medicine, Tripterygium wilfordii, was found to restore cytosolic Ca2+ release in Pkd1−/− murine kidney epithelial cells, by acting as a PC2 agonist. Through this mechanism, triptolide has been effective in arresting cellular proliferation and attenuating overall cyst formation in this murine model (102,103). In a recent pilot study, triptolide was effective in decreasing proteinuria in ADPKD patients but there were no effects on TKV and renal function (104). A clinical trial conducted in China has been terminated due to a high rate of drop-outs [NCT00801268], and another trial is under way [NCT02115659].
Raf kinase inhibitors
Sorafenib is a nonselective Raf inhibitor that decreases ERK activity and inhibits the proliferation of various human cancer cell lines. In an experimental model, sorafenib reduced the basal activity of ERK, inhibited cAMP-dependent activation of B-Raf and MEK/ERK signaling, and caused a concentration-dependent inhibition of cell proliferation induced by cAMP and EGF. In addition, sorafenib completely blocked in vitro cyst growth of human ADPKD cystic cells (105). Conversely, in a PC2 defective mice model, sorafenib inhibited B-Raf but paradoxically activated Raf-1, resulting in an increased ERK1/2 phosphorylation, cell proliferation, and cyst growth in vivo. This effect has been interrupted in the same study by co-administration of sorafenib and octreotide with consequent simultaneous blocking of the cAMP/PKA pathway (106). A different Raf inhibitor (PLX5568) has been evaluated in the Han:SPRD rat model (107). In this study, cyst enlargement attenuated without an improvement in kidney function. Furthermore, the authors reported increased renal and liver fibrosis.
CDK inhibitors
The central role of cyclin-dependent kinases (CDKs) in the regulation of cell proliferation is well defined. PC1 directly regulates cell cycle by inhibiting CDK2 activity through upregulation of p21, inducing cell cycle arrest in G0/G1 phase and controlling terminal differentiation of tubular epithelial cells (108). PC2 by binding with Id2 protein (Inhibitor of DNA binding 2 protein) prevents its translocation to the nucleus, thus blocking cell cycle progression. In ADPKD, translocation of Id2 is connected with downregulation of p21, leading to intensification of CDK2 activity and cell cycle progression (109). A preclinical study with the CKD inhibitor R-roscovitine in juvenile cystic kidney and congenital polycystic kidney mouse models of PKD, effectively attenuated cystogenesis by inhibiting cell cycle progression, proliferation, and apoptosis (110). In addition, a more potent second-generation analogue of roscovitine (S-CR8) showed effective inhibition of both renal and hepatic cystogenesis in an orthologous mouse model of ADPKD with inactivated Pkd1 gene (111).
HDACs inhibitors
Histone deacetylases (HDACs) are part of a vast family of enzymes that regulate specific cellular processes through deacetylation of histones or nonhistone transcription factors, leading to transcriptional repression. Altered expression of HDCAs causes abnormal transcription of key genes controlling principal cellular functions such as cell proliferation, cell-cycle regulation, and apoptosis (112). To discover potential drug candidates in the therapy of ADPKD, a pan-HDAC inhibitor called trichostatin A (TSA) has been evaluated in a pkd2 zebrafish model showing the ability to suppress pronephric cyst formation (113). The same results have been obtained after administration of valproic acid (VPA), a class I HDAC inhibitor (113), and confirmed in Pkd1 and Pkd2 mouse models (113–115). SIRT1 (Sirtuin 1) is a member of a mammalian family of proteins, the sirtuins, originally identified as a family of nicotinamide adenine dinucleotide–dependent (NAD-dependent) class III histone deacylases (116). Zhou et al. (117) showed that SIRT1 expression was upregulated through c-MYC oncoprotein and could be induced by TNF-α, which is present in cyst fluid during cyst development. Double conditional knockouts of Pkd1 and SIRT1 demonstrated delayed renal cyst formation. Increased SIRT1 expression in mutant renal epithelial cells regulated cystic epithelial cell proliferation through deacetylation and phosphorylation of Rb and regulated cystic epithelial cell death through deacetylation of p53. Furthermore, treatment with the pan-sirtuin inhibitor nicotinamide (NAM) or a SIRT1-specific inhibitor (EX-527) delayed cyst growth. An uncontrolled, Open-Label, Pilot and Feasibility Study of Niacinamide in Polycystic Kidney Disease (NIAC-PKD1) [NCT02140814] has just been completed, and we are currently awaiting publication of the results. In addition, another trial, the Pilot Study of Niacinamide in Polycystic Kidney Disease (NIAC-PKD2) [NCT02558595], is currently recruiting participants.
CFTR and KCa3.1 channel inhibitors
The CFTR gene, identified in the q21-31 region of chromosome 7 encodes a cAMP/PKA-regulated Cl− channel (118). CFTR is an ATP-binding cassette (ABC) transporter composed of two transmembrane domains (TMDs) and two nucleotide-binding domains (NBDs), separated by a larger regulatory domain (RD) containing multiple phosphorylation sites. Prior to channel opening, the RD is phosphorylated at multiple sites by PKA (119). CFTR mRNA is expressed in all nephron segments and its protein is involved with chloride secretion in the distal tubule, and the principal cells of the cortical and medullary collecting ducts. Several studies showed that CFTR does not only transport Cl− but also secretes ATP and controls other conductances such as Na+ (ENaC) and K+ (ROMK2) channels (120). As aforementioned, the elevation of intracellular cAMP in ADPKD activates the cAMP/PKA pathway and leads to a consequent accumulation of fluid within cysts by CFTR-mediated transepithelial ion transport (17,18). Furthermore, individuals afflicted by both CFTR and PKD mutations showed to have attenuated polycystic kidney disease phenotype than those with ADPKD alone (121–123). Based on these data, each of the three chemical classes of CFTR inhibitors has been tested in PKD models: (1) Thiazolidinones, (2) Glycine and Malonic Acid Hydrazides, and (3) pyrimido-pyrrolo-quinoxalinediones (PPQs). The best thiazolidinone, tetrazolo-CFTRinh-172, and the best glycine hydrazide, Ph-GlyH-101, were found to inhibit cyst formation and enlargement in MDCK (Madin–Darby Canine Kidney) cyst models and in Pkd1 mice (124). In addition, the PPQ-class CFTR inhibitors PPQ-102 and BPO-27 showed greater potency than the thiazolidinones and glycine hydrazides in embryonic kidney explant PKD models (125,126). The channels for potassium, KCa3.1, located on the basolateral membrane of cells lining the cysts, have also an important role in the Cl− and fluid secretion as mediate K+ efflux and maintain a negative intracellular membrane potential which indirectly enhances apical Cl− secretion by the CFTR. For confirmation, a specific KCa3.1 channel inhibitor, TRAM-34, showed to inhibit Cl− secretion and cyst formation by MDCK cells (127). In conclusion, apart from experimental studies, currently there are no clinical trials to confirm the use of CFTR e/or KCa3.1 inhibitors in clinical practice.
Activation of AMPK signaling pathway
AMP-activated protein kinase (AMPK) is an extremely preserved metabolic sensor of intracellular adenosine nucleotide levels that is activated when even modest decreases in ATP production result in relative increases in AMP or ADP, allowing for adaptive changes in growth, differentiation, and metabolism under conditions of low energy. The most well-described mechanism by which AMPK regulates cell growth is via suppression of the mTORC1 pathway, by direct phosphorylation of the tumor suppressor TSC2 and Raptor (regulatory associated protein of mTOR) (128,129). In addition, AMPK phosphorylates and directly inhibits CFTR in the kidney (130,131). Thus, targeting the activation of AMPK signaling pathway in ADPKD could be useful to arrest two major mechanisms of cystogenesis. Metformin, a drug widely used clinically for diabetes mellitus is known to stimulate AMPK (132,133). Recently, Takiar et al. (134) showed, in Pkd1 mice model, that metformin inhibited renal cystogenesis and caused a significant decrease in the cystic index by activating AMPK and suppressing mTOR and CFTR. Currently, the TAME trial [NCT02656017] is recruiting participants to see if metformin is safe and well tolerated compared with placebo in adult ADPKD patients with beginning stages of chronic kidney disease. The investigators will also measure the effect of metformin on progression of kidney disease as reflected in the kidney size and the kidney function, along with its effect on kidney pain and quality of life.
Agonists of PPAR-γ
Agonists of Peroxisome proliferator–activated receptor gamma (PPAR-γ) are synthetic ligands, used in clinical practice as agents that increase insulin sensitivity in the treatment of diabetes mellitus type II. In addition, they have been shown to have anti-cystogenic properties in PKD animal models (135–138). Pioglitazone has been shown to inhibit the growth of renal and hepatic cysts in PCK rats, by inhibiting the CFTR-mediated ionic current and the secretion of fluid (135). In another study, pioglitazone also reduced cellular proliferation, highlighted by a reduction in the number of cells positive for Ki67 (a proliferation marker) in the dilated tubules and in cysts from treated rats. The fact that it also reduced the number of positive cells for Ki67 in noncystic tubular cells suggest that pioglitazone can inhibit the earliest events of cystic formation (136). Inhibition of renal cell proliferation was mediated by the reduction of two of the crucial intracellular pathways in PKD: the signals MEK/ERK and AKT/mTOR. The inhibitory effect on proliferation was similar in the liver with a reduced rate of the same proliferation markers. Another powerful agonist of PPAR-γ, rosiglitazone has been used to treat Han:SPRD rats. Rosiglitazone delayed the onset of renal failure but was associated with cardiac enlargement due to excessive renal sodium reabsorption (138). Based on these preclinical data, The Use of Low Dose Pioglitazone to Treat Autosomal Dominant Polycystic Kidney Disease (PIOPKD) trial [NCT02697617] is currently recruiting participants to determine whether pioglitazone is a safe and effective treatment of ADPKD when treated in its early stages.
Other drugs have been evaluated in preclinical studies without further investigation through clinical trials. In a seminal study, Natoli et al. (139) showed that the glucosylceramide inhibitor, Genz-123346, was able to block cell cycle progression and proliferation through inhibition of the Akt-mTOR pathway in mouse models for ADPKD and nephronophthisis. Subsequently, the same authors showed that a mutation in the synthase gene of ganglioside GM3 led to a milder cystic phenotype (140). These data suggest that sphingolipids are not only components of cell membranes but also play signaling roles in ADPKD cystogenesis.
Some authors hypothesized that activation of purinergic receptors by ATP modulates fluid secretion, cell proliferation, apoptosis and ciliary function in ADPKD (141,142). Chang et al. (143) showed that the blockade of the purinergic receptor P2X7 by a selective inhibitor reduced cyst formation in a PKD2 zebrafish model, suggesting that P2X7 antagonists could have therapeutic role in ADPKD.
Another pathway that could be implicated in the pathogenetic process of cystogenesis is the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. JAK/STAT indeed plays a relevant role in kidney development and mediates tubular cell proliferation after ischemic injury (28,30). Indeed, pyrimethamine, a STAT3 inhibitor, has been effective in inhibiting cyst growth in PKD1 mice model (30). Subsequently, a STAT3 specific inhibitor (S3I-201) confirmed these beneficial effects (30).
Curcumin (diferuloylmethane) a component of the golden spice turmeric (Curcuma longa) can modulate multiple cell signaling pathways (mTOR, WNT, STAT3) altered in ADPKD and showed to reduce cystogenesis and postpone renal failure in Pkd1 mice model (29).
Conclusion and Future Directions
Multiple signaling pathways are involved in cyst formation and progression, and studies of these signaling pathways have led to potential treatments for ADPKD. In this review, we covered the successes that the scientific society obtained in recent years in understanding the pathogenesis of ADPKD, and presented novel therapeutic strategies targeting molecular pathways of cystogenesis. V2R antagonists and SST analogues have been shown to safely slow kidney growth and protect renal function in patients with ADPKD and represent the most well-characterized and promising candidate therapies to date. According to the results of the TEMPO3/4 study and registration by EMA, tolvaptan seems to be the first-choice drug. Unfortunately, some medical interventions successful in experimental models failed in clinical practice, and others still need to be evaluated in clinical trials. It is possible that monotherapy may not be sufficient and that targeting multiple molecular pathways will be required to retard cyst growth and disease progression in the future. Combination therapy is then the right direction for further clinical trials in order to find effective treatment.
Acknowledgement
The authors acknowledge Dr. Stefanos Tsalouchos for his assistance and for drawing Figure 1.
Conflict of interest
The authors declare no conflicts of interest with respect to research, authorship, and/or publication of this article.
References
-
Chebib FT, Torres VE. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis. 2016;67(5):792–810. http://dx.doi.org/10.1053/j.ajkd.2015.07.037
-
Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149–68. http://dx.doi.org/10.1038/ki.2009.128
-
Rysz J, Gluba-Brzózka A, Franczyk B, Banach M, Bartnicki P. Combination drug versus monotherapy for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Pharmacother. 2016;17(15):2049–56. http://dx.doi.org/10.1080/14656566.2016.1232394
-
Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, et al. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(21) homeostasis in polycystic kidney disease. Biochem Biophys Res Commun. 2001;282(1):341–50. http://dx.doi.org/10.1006/bbrc.2001.4554
-
Anyatonwu GI, Ehrlich BE. Organic cation permeation through the channel formed by polycystin-2. J Biol Chem. 2005;280(33):29488–93. http://dx.doi.org/10.1074/jbc.M504359200
-
LaRiviere WB, Irazabal MV, Torres VE. Novel therapeutic approaches to autosomal dominant polycystic kidney disease. Transl Res. 2015;165(4):488–98. https://dx.doi.org/10.1016/j.trsl.2014.11.003
-
Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs. 2010;19(3):315–28. http://dx.doi.org/10.1517/13543781003588491
-
Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87(6):979–87. http://dx.doi.org/10.1016/S0092-8674(00)81793-6
-
Brasier JL, Henske EP. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest. 1997;99(2):194–9. http://dx.doi.org/10.1172/JCI119147
-
Saigusa T, Bell PD. Molecular pathways and therapies in autosomal-dominant polycystic kidney disease. Physiology (Bethesda). 2015;30(3):195–207. http://dx.doi.org/10.1152/physiol.00032.2014
-
Mochizuki T, Tsuchiya K, Nitta K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol. 2013;17(3):317–26. http://dx.doi.org/10.1007/s10157-012-0741-0
-
Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol. 2014;25(1):18–32. http://dx.doi.org/10.1681/ASN.2013040398
-
Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17(1):178–87. http://dx.doi.org/10.1681/ASN.2005060645
-
Paavola J, Schliffke S, Rossetti S, Kuo IY, Yuan S, Sun Z, et al. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol. 2013;58:199–208. http://dx.doi.org/10.1016/j.yjmcc.2013.01.015
-
Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, et al. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort: renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64(3):1035–45. http://dx.doi.org/10.1046/j.1523-1755.2003.00185.x
-
Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1(1):148–57. http://dx.doi.org/10.2215/CJN.00330705
-
Brill SR, Ross KE, Davidow CJ, Ye M, Grantham JJ, Caplan MJ. Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc Natl Acad Sci U S A. 1996;93(19):10206–11. http://dx.doi.org/10.1073/pnas.93.19.10206
-
Hanaoka K, Devuyst O, Schwiebert EM, Wilson PD, Guggino WB. A role for CFTR in human autosomal dominant polycystic kidney disease. Am J Physiol. 1996;270(1 pt 1):C389–99.
-
Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, et al. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal regulated kinase pathway. Kidney Int. 2000;57(4):1460–71. http://dx.doi.org/10.1046/j.1523-1755.2000.00991.x
-
Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11(7):1179–87.
-
Sweeney WE Jr, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol. 2008;19(7):1331–41. http://dx.doi.org/10.1681/ASN.2007060665
-
Aguiari G, Bizzarri F, Bonon A, Mangolini A, Magri E, Pedriali M, et al. Polycystin-1 regulates amphiregulin expression through CREB and AP1 signalling: implications in ADPKD cell proliferation. J Mol Med (Berl). 2012;90(11):1267–82. http://dx.doi.org/10.1007/s00109-012-0902-3
-
Distefano G, Boca M, Rowe I, Wodarczyk C, Ma L, Piontek KB, et al. Polycystin-1 regulates extracellular signal regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol. 2009;29(9):2359–71. http://dx.doi.org/10.1128/MCB.01259-08
-
Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology. 2010;51(5):1778–88. http://dx.doi.org/10.1002/hep.23511
-
Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of GSK3beta. Mol Cell Biol. 2000;20(24):9356–63. http://dx.doi.org/10.1128/MCB.20.24.9356-9363.2000
-
Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281(15):9971–6. http://dx.doi.org/10.1074/jbc.M508778200
-
Qin S, Taglienti M, Cai L, Zhou J, Kreidberg JA. c-Met and NF-kB-dependent overexpression of Wnt7a and -7b and Pax2 promotes cystogenesis in polycystic kidney disease. J Am Soc Nephrol. 2012;23(8):1309–18. http://dx.doi.org/10.1681/ASN.2011030277
-
Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, et al. Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A. 2011;108(19):7985–90. http://dx.doi.org/10.1073/pnas.1103816108
-
Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, et al. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol. 2011;300(5):F1193–202. http://dx.doi.org/10.1152/ajprenal.00419.2010
-
Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, et al. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet. 2011;20(21):4143–54. http://dx.doi.org/10.1093/hmg/ddr338
-
Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372(14):1349–58. http://dx.doi.org/10.1056/NEJMra1404726
-
Meijer E, Bakker SJL, van der Jagt EJ, Navis G, de Jong PE, Struck J, et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(2):361–8. http://dx.doi.org/10.2215/CJN.04560510
-
Meijer E, Boertien WE, Zietse R, Gansevoort RT. Potential deleterious effects of vasopressin in chronic kidney disease and particularly autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2011;34(4):235–44. http://dx.doi.org/10.1159/000326902
-
Boertien WE, Meijer E, Zittema D, van Dijk MA, Rabelink TJ, Breuning MH, et al. Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2012;27(11):4131–7. http://dx.doi.org/10.1093/ndt/gfs070
-
Zittema D, Boertien WE, van Beek AP, Dullaart RPF, Franssen CFM, de Jong PE, et al. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol. 2012;7(6):906–13. http://dx.doi.org/10.2215/CJN.11311111
-
Ahrabi AK, Terryn S, Valenti G, Caron N, Serradeil-Le Gal C, Raufaste D, et al. PKD1 haploinsufficiency causes a syndrome of inappropriate antidiuresis in mice. J Am Soc Nephrol. 2007;18(6):1740–53. http://dx.doi.org/10.1681/ASN.2006010052
-
Ho TA, Godefroid N, Gruzon D, Haymann J-P, Maréchal C, Wang X, et al. Autosomal dominant polycystic kidney disease is associated with central and nephrogenic defects in osmoregulation. Kidney Int. 2012;82(10):1121–9. http://dx.doi.org/10.1038/ki.2012.225
-
Yasuda G, Jeffries WB. Regulation of cAMP production in initial and terminal inner medullary collecting ducts. Kidney Int. 1998;54(1):80–6. http://dx.doi.org/10.1046/j.1523-1755.1998.00990.x
-
Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17(8):2220–7. http://dx.doi.org/10.1681/ASN.2006030251
-
Gattone VH 2nd, Maser RL, Tian C, Rosenberg JM, Branden MG. Developmental expression of urine concentration-associated genes and their altered expression in murine infantile-type polycystic kidney disease. Dev Genet. 1999;24(3–4):309–18. http://dx.doi.org/10.1002/(SICI)1520-6408(1999)24:3/4<309::AID-DVG14>3.0.CO;2-5
-
Gattone VH 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9(10):1323–6. http://dx.doi.org/10.1038/nm935
-
Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH II. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10(4):363–4. http://dx.doi.org/10.1038/nm1004
-
Meijer E, Gansevoort RT, de Jong PE, van der Wal AM, Leonhard WN, de Krey SR, et al. Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: optimal timing and dosing of the drug. Nephrol Dial Transplant. 2011;26(8):2445–53. http://dx.doi.org/10.1093/ndt/gfr069
-
Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287(3):860–7.
-
Wang X, Gattone V 2nd, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat, J Am Soc Nephrol. 2005;16(4):846–51. http://dx.doi.org/10.1681/ASN.2004121090
-
Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation, Cl secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol. 2011;301(5):F1005–13. http://dx.doi.org/10.1152/ajprenal.00243.2011
-
Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19(1):102–8. http://dx.doi.org/10.1681/ASN.2007060688
-
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, et al. TEMPO 3:4 Trial Investigators: tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–18. https://dx.doi.org/10.1056/NEJMoa1205511
-
Irazabal MV, Torres VE, Hogan MC, Glockner J, King BF, Ofstie TG, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301. http://dx.doi.org/10.1038/ki.2011.119
-
Boertien WE, Meijer E, de Jong PE, ter Horst GJ, Renken RJ, van der Jagt EJ, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65(6):833–41. http://dx.doi.org/10.1053/j.ajkd.2014.11.010
-
Boertien WE, Meijer E, de Jong PE, Bakker SJ, Czerwiec FS, Struck J, et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84(6):1278–86. http://dx.doi.org/10.1038/ki.2013.285
-
Gansevoort RT, Meijer E, Chapman AB, Czerwiec FS, Devuyst O, Grantham JJ, et al. TEMPO 3:4 Investigators. Nephrol Dial Transplant. 2016;31(11):1887–94. http://dx.doi.org/10.1093/ndt/gfv422
-
Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, et al. Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3–4 study. Am J Kidney Dis. 2011;57(5):692–9. http://dx.doi.org/10.1053/j.ajkd.2010.11.029
-
Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, et al. Tolvaptan in autosomal dominant polycystic kidney disease: three years’ experience. Clin J Am Soc Nephrol. 2011;6(10):2499–507. http://dx.doi.org/10.2215/CJN.03530411
-
Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016;31(3):337–48. http://dx.doi.org/10.1093/ndt/gfv456
-
Renal Association Working Group on Tolvaptan in ADPKD. http://www.renal.org/docs/default-source/guidelines-resources/other-guidlines/tolvaptan_in_adpkd-rawg2015_commentary-060516clean.pdf?sfvrsn=0
-
Devuyst O, Chapman AB, Boklage S. Tolerability of aquaretic-related symptoms: results from the TEMPO 3:4 trial [abstract plus poster]. World Congress of Nephrology; 2015. http://www.wcn2015.org
-
European Medicines Agency. Tolvaptan (Jinarc): summary of product characteristics; 2015. http://www.ema.europa.eu [Accessed 18 Sep 2015].
-
Otsuka Pharmaceutical Development and Commercialization. Open-label trial to evaluate the long term safety of titrated immediate-release tolvaptan in subjects with autosomal dominant polycystic kidney disease. 2014. http://www.clinicaltrials.gov/ct2/show/NCT02251275 [Accessed 12 Feb 2017]
-
Otsuka Pharmaceutical Development and Commercialization. Efficacy and safety of tolvaptan in subjects with chronic kidney disease between late stage 2 to early stage 4 due to autosomal dominant polycystic kidney disease. 2014. http://www.clinicaltrials.gov/ct2/show/NCT02160145 [Accessed 12 Feb 2017]
-
Rai U, Thrimawithana TR, Valery C, Young SA. Therapeutic uses of somatostatin and its analogues: current view and potential applications. Pharmacol Ther. 2015;152:98–110. http://dx.doi.org/10.1016/j.pharmthera.2015.05.007
-
Balster DA, O’Dorisio MS, Summers MA, Turman MA. Segmental expression of somatostatin receptor subtypes sst(1) and sst(2) in tubules and glomeruli of human kidney. Am J Physiol Renal Physiol. 2001;280(3):F457–65.
-
Bates CM, Kegg H, Grady S. Expression of somatostatin in the adult and developing mouse kidney. Kidney Int. 2004;66(5):1785–93. http://dx.doi.org/10.1111/j.1523-1755.2004.00953.x
-
Bates CM, Kegg H, Grady S. Expression of somatostatin receptors 1 and 2 in the adult mouse kidney. Regul Pept. 2004;119(1–2):11–20. http://dx.doi.org/10.1016/j.regpep.2003.12.015
-
Bhandari S, Watson N, Long E, Sharpe S, Zhong W, Xu SZ, et al. Expression of somatostatin and somatostatin receptor subtypes 1–5 in human normal and diseased kidney. J Histochem Cytochem. 2008;56(8):733–43. https://dx.doi.org/10.1369/jhc.2008.950998
-
Friedlander G, Amiel C. Somatostatin and alpha 2-adrenergic agonists selectively inhibit vasopressin-induced cyclic AMP accumulation in MDCK cells. FEBS Lett. 1986;198(1):38–42. http://dx.doi.org/10.1016/0014-5793(86)81180-2
-
Winkler SN, Torikai S, Levine BS, Kurokawa K. Effect of somatostatin on vasopressin-induced antidiuresis and renal cyclic AMP of rats. Miner Electrolyte Metab. 1982;7(1):8–14.
-
Tan CK, Podila PV, Taylor JE, Nagorney DM, Wiseman GA, Gores GJ, et al. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology. 1995;108(6):1908–16. http://dx.doi.org/10.1016/0016-5085(95)90157-4
-
Irazabal MV, Torres VE. Experimental therapies and ongoing clinical trials to slow down progression of ADPKD. Curr Hypertens Rev. 2013;9(1):44–59. http://dx.doi.org/10.2174/1573402111309010008
-
Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68(1):206–16. http://dx.doi.org/10.1111/j.1523-1755.2005.00395.x
-
Caroli A, Antiga L, Cafaro M, Fasolini G, Remuzzi A, Remuzzi G, et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5(5):783–9. http://dx.doi.org/10.2215/CJN.05380709
-
Tietz Bogert PS, Huang BQ, Gradilone SA, Masyuk TV, Moulder GL, Ekker SC, et al. The zebrafish as a model to study polycystic liver disease. Zebrafish. 2013;10(2):211–17. http://dx.doi.org/10.1089/zeb.2012.0825
-
Hopp K, Hommerding CJ, Wang X, Ye H, Harris PC, Torres VE. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol. 2015;26(1):39–47. http://dx.doi.org/10.1681/ASN.2013121312
-
van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137(5):1661-8.e1-2.
-
Chrispijn M, Nevens F, Gevers TJ, Vanslembrouck R, van Oijen MG, Coudyzer W. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther. 2012;35(2):266–74. http://dx.doi.org/10.1111/j.1365-2036.2011.04923.x
-
Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052–61. http://dx.doi.org/10.1681/ASN.2009121291
-
Hogan MC, Masyuk TV, Page L, Holmes DR 3rd, Li X, Bergstralh EJ, et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27(9):3532–9. http://dx.doi.org/10.1093/ndt/gfs152
-
Caroli A, Perico N, Perna A, Antiga L, Brambilla P, Pisani A, et al. Effect of long acting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet (London, England). 2013;382(9903):1485–95. http://dx.doi.org/10.1016/S0140-6736(13)61407-5
-
Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Wetzels JF, Drenth JP. Effect of lanreotide on polycystic liver and kidneys in autosomal dominant polycystic kidney disease: an observational trial. Liver Int. 2015;35(5):1607–14. http://doi.org/10.1111/liv.12726
-
Myint TM, Rangan GK, Webster AC. Treatments to slow progression of autosomal dominant polycystic kidney disease: systematic review and meta-analysis of randomized trials. Nephrology (Carlton). 2014;19(4):217–26. http://dx.doi.org/10.1111/nep.12211
-
Lantinga MA, D’Agnolo HM, Casteleijn NF, de Fijter JW, Meijer E, Messchendorp AL, et al. Hepatic Cyst Infection During Use of the Somatostatin Analog Lanreotide in Autosomal Dominant Polycystic Kidney Disease: an Interim Analysis of the Randomized Open-Label Multicenter DIPAK-1 Study. Drug Saf. 2017; 40(2):153–167.
-
Ibraghimov-Beskrovnaya O, Natoli TA. mTOR signaling in polycystic kidney disease. Trends Mol Med. 2011;17(11):625–33. http://dx.doi.org/10.1016/j.molmed.2011.06.003
-
Brook-Carter PT, Peral B, Ward CJ, Thompson P, Hughes J, Maheshwar MM, et al. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease – A contiguous gene syndrome. Nat Genet. 1994;8(4):328–32. http://dx.doi.org/10.1038/ng1294-328
-
Cai SL, Walker CL. TSC2, a key player in tumor suppression and cystic kidney disease. Nephrol Ther. 2006;2 Suppl 2:S119–22.
-
Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell Biol. 2012;47(4):535–46. http://dx.doi.org/10.1016/j.molcel.2012.06.009
-
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103(14):5466–71. http://dx.doi.org/10.1073/pnas.0509694103
-
Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol. 2010;21(3):489–97. http://dx.doi.org/10.1681/ASN.2009040421
-
Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant. 2006;21(3):598–604. http://dx.doi.org/10.1093/ndt/gfi181
-
Wu M, Wahl PR, Le Hir M, Wackerle-Men Y, Wuthrich RP, Serra AL, et al. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30(4):253–9. http://dx.doi.org/10.1159/000104818
-
Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, et al. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-Mtor activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst. 2008;100(2):140–54. http://dx.doi.org/10.1093/jnci/djm288
-
Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16(1):46–51. http://dx.doi.org/10.1681/ASN.2004080660
-
Zafar I, Belibi FA, He Z, Edelstein CL. Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD). Nephrol Dial Transplant. 2009;24(8):2349–53. http://dx.doi.org/10.1093/ndt/gfp129
-
Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):830–40. http://dx.doi.org/10.1056/NEJMoa1003491
-
Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):820–9. http://dx.doi.org/10.1056/NEJMoa0907419
-
Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, et al. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol. 2010;21(6):1031–40. http://dx.doi.org/10.1681/ASN.2009121302
-
Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol Dial Transplant. 2012;27(9):3560–7. http://dx.doi.org/10.1093/ndt/gfs264
-
Braun WE, Schold JD, Stephany BR, Spirko RA, Herts BR. Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study. Clin J Am Soc Nephrol. 2014;9(5):881–8. http://dx.doi.org/10.2215/CJN.02650313
-
Moes DJ, Guchelaar HJ, de Fijter JW. Sirolimus and everolimus in kidney transplantation. Drug Discov Today. 2015;20(10):1243–9. http://dx.doi.org/10.1016/j.drudis.2015.05.006
-
Musumeci F, Schenone S, Brullo C, Botta M. An update on dual Src/Abl inhibitors. Future Med Chem. 2012;4(6):799–822. http://dx.doi.org/10.4155/fmc.12.29
-
Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004;279(39):40419–30. http://dx.doi.org/10.1074/jbc.M405079200
-
Elliott J, Zheleznova NN, Wilson PD. c-Src inactivation reduces renal epithelial cell-matrix adhesion, proliferation, and cyst formation. Am J Physiol Cell Physiol. 2011;301(2):C522–9. http://dx.doi.org/10.1152/ajpcell.00163.2010
-
Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104(11):4389–94. http://dx.doi.org/10.1073/pnas.0700499104
-
Leuenroth SJ, Bencivenga N, Chahboune H, Hyder F, Crews CM. Triptolide reduces cyst formation in a neonatal to adult transition Pkd1 model of ADPKD. Nephrol Dial Transplant. 2010;25(7):2187–94. http://dx.doi.org/10.1093/ndt/gfp777
-
Chen D, Ma Y, Wang X, Yu S, Li L, Dai B, et al. Triptolide-containing formulation in patients with autosomal dominant polycystic kidney disease and proteinuria: an uncontrolled trial. Am J Kidney Dis. 2014;63(6):1070–2. http://dx.doi.org/10.1053/j.ajkd.2014.01.418
-
Yamaguchi T, Reif GA, Calvet JP, Wallace DP. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2010;299(5):F944–51. http://dx.doi.org/10.1152/ajprenal.00387.2010
-
Spirli C, Morell CM, Locatelli L, Okolicsanyi S, Ferrero C, Kim AK, et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology. 2012;56(6):2363–74. http://dx.doi.org/10.1002/hep.25872
-
Buchholz B, Klanke B, Schley G, Bollag G, Tsai J, Kroening S. The Raf kinase inhibitor PLX5568 slows cyst proliferation in rat polycystic kidney disease but promotes renal and hepatic fibrosis. Nephrol Dial Transplant. 2011;26(11):3458–65. http://dx.doi.org/10.1093/ndt/gfr432
-
Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109(2):157–68. http://dx.doi.org/10.1016/S0092-8674(02)00716-X
-
Li X, Luo Y, Starremans PG, McNamara CA, Pei Y, Zhou J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat Cell Biol. 2005;7(12):1202–12. http://dx.doi.org/10.1038/ncb1326
-
Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444(7121):949–52. http://dx.doi.org/10.1038/nature05348
-
Bukanov NO, Moreno SE, Natoli TA, Rogers KA, Smith LA, Ledbetter SR, et al. CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD. Cell Cycle. 2012;11(21):4040–6. http://dx.doi.org/10.4161/cc.22375
-
Chen HP, Zhao YT, Zhao TC. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog. 2015;20(1–2):35–47. http://dx.doi.org/10.1615/CritRevOncog.2015012997
-
Cao Y, Semanchik N, Lee SH, Somlo S, Barbano PE, Coifman R, et al. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci U S A. 2009;106(51):21819–24. http://dx.doi.org/10.1073/pnas.0911987106
-
Xia S, Li X, Johnson T, Seidel C, Wallace DP, Li R. Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development. 2010;137(7):1075–84. http://dx.doi.org/10.1242/dev.049437
-
Fan LX, Li X, Magenheimer B, Calvet JP, Li X. Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int. 2012;81(1):76–85. http://dx.doi.org/10.1038/ki.2011.296
-
Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25(3):138–45. http://dx.doi.org/10.1016/j.tem.2013.12.001
-
Zhou X, Fan LX, Sweeney WE Jr, Denu JM, Avner ED, Li X. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J Clin Invest. 2013;123(7):3084–98. http://dx.doi.org/10.1172/JCI64401
-
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–73. http://dx.doi.org/10.1126/science.2475911
-
Akabas MH. Cystic fibrosis transmembrane conductance regulator. Structure and function of an epithelial chloride channel. J Biol Chem. 2000;275(6):3729–32. http://dx.doi.org/10.1074/jbc.275.6.3729
-
Morales MM, Falkenstein D, Lopes AG. The cystic fibrosis transmembrane regulator (CFTR) in the kidney. An Acad Bras Cienc. 2000;72(3):399–406. http://dx.doi.org/10.1590/S0001-37652000000300013
-
O’Sullivan DA, Torres VE, Gabow PA, Thibodeau SN, King BF, Bergstralh EJ. Cystic fibrosis and the phenotypic expression of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32(6):976–83. http://doi.org/10.1016/S0272-6386(98)70072-1
-
Persu A, Devuyst O, Lannoy N, Materne R, Brosnahan G, Gabow PA, et al. CF gene and cystic fibrosis transmembrane conductance regulator expression in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11(12):2285–96.
-
Xu N, Glockner JF, Rossetti S, Babovich-Vuksanovic D, Harris PC, Torres VE. Autosomal dominant polycystic kidney disease coexisting with cystic fibrosis. J Nephrol. 2006;19(4):529–34.
-
Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1300–10. http://dx.doi.org/10.1681/ASN.2007070828
-
Tradtrantip L, Sonawane ND, Namkung W, Verkman AS. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem. 2009;52(20):6447–55. http://dx.doi.org/10.1021/jm9009873
-
Snyder DS, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J Med Chem. 2011;54(15):5468–77. http://dx.doi.org/10.1021/jm200505e
-
Albaqumi M, Srivastava S, Li Z, Zhdnova O, Wulff H, Itani O, et al. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;74(6):740–9. http://dx.doi.org/10.1038/ki.2008.246
-
Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;26;115(5):577–90. http://dx.doi.org/10.1016/S0092-8674(03)00929-2
-
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–26. http://dx.doi.org/10.1016/j.molcel.2008.03.003
-
Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000;105(12):1711–21. http://dx.doi.org/10.1172/JCI9622
-
King JD Jr, Fitch AC, Lee JK, McCane JE, Mak DO, Foskett JK. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol. 2009;297(1):C94–101. http://dx.doi.org/10.1152/ajpcell.00677.2008
-
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. http://dx.doi.org/10.1172/JCI13505
-
Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–6. http://dx.doi.org/10.1126/science.1120781
-
Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A. 2011;108(6):2462–7. http://dx.doi.org/10.1073/pnas.1011498108
-
Blazer-Yost BL, Haydon J, Eggleston-Gulyas T, Chen JH, Wang X, Gattone V, et al. Pioglitazone attenuates cystic burden in the PCK rodent model of polycystic kidney disease. PPAR Res. 2010;2010:274376. http://dx.doi.org/10.1155/2010/274376
-
Yoshihara D, Kurahashi H, Morita M, Kugita M, Hiki Y, Aukema HM, et al. PPAR-gamma agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300(2):F465–74. http://dx.doi.org/10.1152/ajprenal.00460.2010
-
Muto S, Aiba A, Saito Y, Nakao K, Nakamura K, Tomita K, et al. Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum Mol Genet. 2002;11(15):1731–42. http://dx.doi.org/10.1093/hmg/11.15.1731
-
Dai B, Liu Y, Mei C, Fu L, Xiong X, Zhang Y, et al. Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin Sci (Lond). 2010;119(8):323–33. http://doi.org/10.1042/CS20100113
-
Natoli TA, Smith LA, Rogers KA, Wang B, Komarnitsky S, Budman Y, et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16(7):788–92. http://dx.doi.org/10.1038/nm.2171
-
Natoli TA, Husson H, Rogers KA, Smith LA, Wang B, Budman Y, et al. Loss of GM3 synthase gene, but not sphingosine kinase 1, is protective against murine nephronophthisis-related polycystic kidney disease. Hum Mol Genet. 2012;21(15):3397–407. http://dx.doi.org/10.1093/hmg/dds172
-
Turner CM, Ramesh B, Srai SK, Burnstock G, Unwin RJ. Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs. 2004;178(3):168–79. http://dx.doi.org/10.1159/000082247
-
Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, et al. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296(6):F1464–76. http://dx.doi.org/10.1152/ajprenal.90542.2008
-
Chang MY, Lu JK, Tian YC, Chen YC, Hung CC, Huang YH. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol. 2011;22(9):1696–706. http://dx.doi.org/10.1681/ASN.2010070728