ORIGINAL ARTICLE
Role of JAK3 in the Pathogenesis of Oxidative Stress-Induced Kidney Fibrosis
Betty Pat1,2, David W. Johnson1,3, Glenda C. Gobe1
1Kidney Disease Research Collaborative, University of Queensland and Princess Alexandra Hospital at the Translational Research Institute, Woolloongabba, Australia; 2The University of Alabama, Birmingham AL, USA; 3Department of Renal Medicine, Princess Alexandra Hospital, Ipswich Road, Woolloongabba, Brisbane, Australia
Abstract
The Janus kinase (JAK) tyrosine kinase family and JAK/STAT signal transduction pathway may act in kidney fibrogenesis. JAK3 expression was investigated in in vitro and in vivo models of kidney fibrosis involving oxidative stress. There was a marked down-regulation of JAK3 mRNA in rat kidney tubular epithelial cells (NRK52E) and fibroblasts (NRK49F) exposed to 1.0 mM H2O2 for 18–20 h compared with controls, which correlated with increased apoptosis and decreased mitosis in both cell lines. However, JAK3 protein levels were not significantly different in control and H2O2-treated epithelial and fibroblast cultures. JAK3 activation (phospho-tyrosine) increased in NRK52E cells and decreased in NRK49F cells with oxidative stress. STAT3 phosphorylation decreased in both cell lines with oxidative stress compared with controls. JAK3 protein expression and localisation were investigated in kidneys using the unilateral ureteral obstruction (UUO) model (0–7 days, rats) of kidney fibrosis that involves oxidative stress. JAK3 protein expression did not differ between UUO and controls; however, JAK3 localisation increased temporally with UUO, with strong epithelial expression in mitotic cells compared with controls. Apoptotic tubular epithelium showed minimal JAK3. In summary, in vitro, decreased kidney JAK3 mRNA after oxidative stress was not seen translationally. Differences in the activation of the JAK3/STAT3 pathway may have different consequences for renal fibrosis. In vivo, changes in JAK3 protein localisation, and especially its co-localisation with mitotic cells, indicate that JAK3 protein may contribute to renal tubular epithelial cell proliferation after oxidative stress.
Keywords: JAK3; Janus kinase tyrosine kinase family; kidney fibrosis; oxidative stress; STAT
Received: 01 March 2018;
Accepted after revision: 09 April 2018;
Published: 14 May 2018
Author for correspondence: Glenda C. Gobe, Kidney Disease Research Collaborative, University of Queensland and Princess Alexandra Hospital at the Translational Research Institute, 37 Kent Street, Woolloongabba, Brisbane, Australia. Email:
g.gobe@uq.edu.auHow to cite: Pat B et al. Role of JAK3 in the pathogenesis of oxidative stress-induced kidney fibrosis. J Ren Hepat Disord. 2018;2(1):18–26.
Doi: http://dx.doi.org/10.15586/jrenhep.2018.30Copyright: Pat B et al.
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
The Janus kinase (JAK) family of signal transduction molecules consist of non-receptor tyrosine kinases, which are activated in the cytoplasm rather than at the cell membrane (1). There are currently four characterised JAK kinases—JAK1, JAK2, JAK3 and TYK2. Nuclear signalling from these kinases is mediated through a group of proteins known as signal transducers and activators of transcription (STAT). Seven members of this family (STAT1-4, STAT5a, STAT5b and STAT6) are tyrosine phosphorylated by JAKs in the cytoplasm and then proceed to translocate into the nucleus where they act as transcription factors (2).
The role of the JAK/STAT signalling pathway has received limited attention in kidney fibrosis, and results regarding its role are disparate. Alterations in the JAK/STAT signalling pathway may mediate signalling initiated by reactive oxygen species, as well as other pro-fibrotic factors such as cytokines, growth factors and angiotensin II (3, 4). Renal fibroblast (NRK49F) proliferation induced by advanced glycation end-products (AGE), often formed after oxidative stress, occurred through a JAK/STAT-dependent pathway (3, 5, 6), but only in association with JAK2 activation and not any of the other members of the JAK family (JAK1, JAK3 or TYK2). Koike et al. (4) reported that JAK/STAT activation had a protective role against kidney fibrosis in the unilateral ureteral obstruction (UUO) model of kidney fibrosis in mice; but, Pang et al. (7) found that use of a STAT3 inhibitor attenuated kidney tubulointerstitial fibrosis in the same rodent model. JAK/STATs were reported to be activated during pro-inflammatory IL-6-induced proliferation in renal cancer cells (8). In a similar theme involving inflammation, Dai et al. (9) found that JAK signalling was activated by peritoneal dialysate in in vitro and in vivo kidney fibrosis models. Finally, Wiezel et al. (10) reported impaired JAK/STAT signalling in chronic kidney disease.
Multiple signalling pathways acting in parallel may be responsible for the various outcomes in renal fibrosis. We and others have shown that signal transduction pathways, such as the mitogen-activated protein kinase (MAPK) pathway, play an integral role in determining renal cell proliferation, death and the propagation of disease states in the fibrosing kidney, especially after oxidative stress in cell culture and in the UUO model in rats (11–13). In the current investigation, initial pilot data from microarray analysis indicated significantly altered (decreased) levels of JAK3, but not JAK1 or JAK2, in kidney fibrogenesis. The purpose of this study was to compare JAK3 expression and activation with pathology in well-established models of renal fibrosis, using in vitro and in vivo models. The interrelationship between JAK3 and extracellular signalling-regulated kinase (ERK) was also investigated in the in vivo model of kidney fibrosis.
Materials and methods
Microarray analysis
Total RNA was extracted from matched serum-free (SF) control and 1.0 mM hydrogen peroxide (H2O2)-treated (18–20 h) cell lines from three separate experiments for cDNA expression profiling. RNA extraction was carried out using the RNeasy Mini Kit (Qiagen) with combined proteinase K digestion as described by the manufacturer. Total RNA (20 μg) from SF RNA (labelled with Cy5) or 1.0 mM H2O2 RNA (labelled with Cy3) (reverse labelling reaction was also performed) was mixed with 40U RNasin (Promega), 4 μg oligo d(T15) and 6 μg random hexamer primers (Invitrogen, Carlsbad, CA) and labelled using the amino-allyl (indirect) method according to the manufacturer’s protocol. Hybridisation was carried out in 20 μg of Cot-1 DNA (Invitrogen), 20 μg of poly dA (Sigma) and 80 μL of DIG Easy Hybridisation solution (Roche Diagnostics, Castle Hill, NSW, Australia) for 14–16 h at 37°C in humidified hybridisation chambers (TeleChem International Inc, Sunnyvale, CA). Microarrays were washed twice in a pre-heated (37°C) solution of 1× saline–sodium citrate (SSC) and 0.1% sodium dodecyl sulphate (SDS) for 5 min, then in 1× SSC for 3 min, and finally in 0.1× SSC for 1 min before drying by centrifugation at 100 g for 5 min. Microarray slides were immediately scanned in a GMS-418 Confocal Scanner (Genetic MicroSystems/Affymetrix Inc, Santa Clara, CA) and images imported into ImaGene 5.0 (BioDiscovery Inc, Marina Del Rey, CA) for data extraction. Mean signal pixel intensities and mean background pixel intensities for Cy3 and Cy5 channels were imported into GeneSpring 6 (Silicon Genetics, Redwood City, CA) and normalised using the Lowess algorithm to correct for intensity-dependent bias and data filtering to remove experimental noise and poor data before further analysis. The microarray chips used in this study were from the Clive and Vera Ramaciotti Centre for Gene Function Analysis (University of New South Wales, Sydney, Australia) containing 5535 oligonucleotides spotted onto 1 microarray slide. Gene expression was calculated as the ratio to the SF RNA. Genes with RNA differentially regulated were further segregated into lists of those that were either upregulated or downregulated by 1.0 mM H2O2 treatment. Genes were selected on the basis of their known involvement as active constituents, regulators or associated components of characterised signal transduction pathways, in addition to their possible involvement in renal fibrogenesis. Of the 5535 genes spotted onto the Ramaciotti MWG oligo sets, image analysis involving background subtraction and data normalisation indicated that 76 and 81 genes were differentially regulated by ≥ a twofold change (P < 0.05) between SF control and 1.0 mM H2O2-treated samples in renal epithelial (NRK52E) and fibroblast (NRK49F) cell lines, respectively. The expression profile of JAK3 indicated that it was downregulated by 1.0 mM H2O2 treatment in both NRK52E and NRK49F cells by fold differences of 4.117 and 6.417, respectively (Table 1). JAK1 and JAK2 had negligible alteration. Thus, JAK3 was selected for the following investigation. RT-PCR was used to confirm that JAK3 cDNA levels were reduced in both cell lines; however, no changes were observed with JAK1 and JAK2 (data not demonstrated).
Table 1. Different mRNA expression profiles occur for JAK1, JAK2 and JAK3 after oxidative stress.
|
JAK1 |
JAK2 |
JAK3 |
| 52E |
No change |
No change |
↓ 4.117 |
| 49F |
No change |
No change |
↓ 6.417 |
| 49F, NRK49F kidney fibroblast cells; 52E, NRK52E kidney epithelial cells. |
In vitro oxidative stress model
Renal fibroblast (NRK49F) and epithelial (NRK52E) cell lines were grown to 90–100% confluence in 25 or 75 cm2 flasks in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% bovine calf serum and 1000 U/mL penicillin and 1000 μg/mL streptomycin. Cells were treated with either DMEM with (control) and without serum (SF), or SF containing 1.0 mM H2O2 for 18–24 h. This treatment concentration was selected because it produces a moderate amount of apoptosis and decreased mitosis in vitro, two characteristics of the intrinsic renal cells in diseases in vivo involving oxidative stress (12). This model has been used in other studies to define expression of many pro-fibrotic molecules (11). Following treatment, cells were analysed for apoptosis and proliferation or trypsinised, collected and pellets stored at –80°C prior to extraction of RNA or protein. For RNA analysis, the process was as described previously. For Western blots, established methods were used (14). In brief, cells were lysed directly on the culture plate in cell lysis buffer (0.15 M NaCl, 0.025 M NaF, 0.5 M EDTA, 0.1% SDS, 1.0% Igepal CA-630 in 50 mM Tris-Cl pH7.5) containing phosphatase and protease inhibitors (10 μg/mL leupeptin, 10 μg/mL aprotinin, 100 μg/mL phenylmethanesulphonyl fluoride, 1 mM sodium vanadate). Cell debris was collected by centrifugation at 16,000 g for 15 min at 4°C and protein concentration in the supernatant determined using a Bradford protein assay (Bio-Rad). Excess protein was aliquoted and stored at −80°C.
UUO in vivo kidney fibrosis model
All experiments were performed with ethics approval from the University of Queensland Animal Experimentation Ethics Committee (Number Path/RBH/732/03/RD). Male Sprague-Dawley rats (N = 6 per group) weighing 180–220 g were used, as previously described, for a period ranging from 0 to 7 days, and results on increasing development of fibrosis over this time have been previously described by us (12). Briefly for UUO, the left kidney was exposed with a mid-abdominal incision and a double ligature placed on the left ureter approximately 1 cm from the renal hilum, before closing the abdominal incision with 4–0 silk and Michel clips. At each time point, each kidney was quickly sliced transversely (2–3 mm slices) to the length of the kidney. The slices closest to the equatorial plane were either fixed in 10% buffered formalin or 4% buffered paraformaldehyde for histology and immunohistochemistry (IHC), or snap frozen in liquid nitrogen for protein or RNA extraction and analysis.
Histology
Apoptosis and mitosis were assessed using morphology (15) following hematoxylin and eosin (H&E) staining and microscopy. Data were obtained by counting 10 frames of cells for controls and treatment and calculating the percentage of apoptotic or mitotic cells. The morphological characteristics for apoptosis were as follows: (i) shrunken eosinophilic cells with condensed, marginated nuclear chromatin and intact cell membrane; (ii) discrete apoptotic bodies comprising large, dense, pyknotic nuclear fragments surrounded by a narrow eosinophilic cytoplasm; and (iii) clusters of small apoptotic bodies (assessed as a single apoptotic occurrence). The morphological characteristics used to distinguish mitosis were as follows: (i) formation of mitotic spindles occurring during metaphase and remaining visible in anaphase or (ii) cells in the later stages of mitosis, telophase or undergoing cytokinesis. Morphological assessment has been confirmed as a reliable measure of apoptosis and mitosis, in a similar experimental protocol using molecular biomarkers for apoptosis (ApopTag) and mitosis (PCNA) in parallel with morphology (16).
Western immunoblots
Disruption of tissue or cells was carried out in ice-cold cell lysis buffer (50 mM Tris-Cl at pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 25 mM NaF, 0.5 M EDTA) containing protease and phosphatase inhibitors (100 μg/mL PMSF, 20 μg/mL leupeptin, 20 μg/mL aprotinin, 1 mM sodium orthovanadate, all Sigma-Aldrich products) using a tissue homogenizer (14). Cell debris was removed by centrifugation at 16,000 g for 15 min at 4°C. Protein concentration was determined in each tissue extract by a Bradford protein assay (Bio-Rad) and spectrophotometry at 595 nm; 40 μg of total protein were electrophoresed on a 10% SDS polyacrylamide gel using a Bio-Rad mini-protean unit, transferred to a polyvinylidene difluoride (PVDF) membrane and blotted routinely with JAK3 (Neomarkers/Lab Vision, Fremont, CA) (1:100), ERK (1:1000) or pERK (1:1000) (both from Cell Signaling Technology, Beverly, MA) primary antibodies and then appropriate horse-radish peroxidase conjugated secondary antibodies diluted (1:2000–1:5000) in 5% powdered milk in Tris-buffered saline. Phosphorylated tyrosine (pTyr) and phosphorylated STAT3 (Invitrogen; 1:400) were analysed for JAK activation. Protein bands were visualised using enhanced chemiluminescence. X-ray film was scanned using a Hewlett Packard ScanJet 3200 C at 300 dpi, and Scion Image (β4.0.2) software was used to quantify the density of the bands in arbitrary densitometry units. Membranes were routinely stained with Coomassie Blue (Sigma-Aldrich), or actin immunoblots were used, to verify equal protein loading of lanes.
IHC and double staining
Sub-serial sections of paraffin-embedded kidney slices were cut, numbered, deparaffinised and rehydrated, before staining with the peroxidase–anti-peroxidase IHC method published by us previously (17). Non-specific binding of peroxidase or antibodies was blocked with 0.3% H2O2 in 4% skim milk powder followed by incubation in diluted normal rabbit or goat serum. Primary antibodies were JAK3 (mouse monoclonal, Neomarkers/Lab Vision, Fremont, CA; 1:100) or phosphorylated ERK (pERK; rabbit polyclonal, New England Biolabs, Beverly, MA, USA; 1:400 dilution) and PCNA (mouse monoclonal, DAKO, Glostrup, Denmark; 1:50 dilution). The appropriate secondary antibody was applied at a dilution of 1:200 to 1:400 in Tris buffer. Sections were developed using the peroxidase–anti-peroxidase reaction plus the chromogen diaminobenzidine tetrahydrochloride (DAB) for IHC. Negative controls were prepared without primary antibody or with non-specific serum. Positive tissue control sections from unrelated studies were included. Sections were lightly counterstained with haematoxylin and then dehydrated in ethanols, cleared in xylene and mounted in Depex.
Double staining
Using paraffin-embedded sections, double antibody IHC was performed with pERK (1:400) and JAK3 (1:100). Staining procedures were identical to previous IHC methods with the exception of the chromogen. For pERK IHC, sections were developed with the NovaRED™ substrate kit for peroxidase detection (red label) (Vector Laboratories; catalogue number SK-4800), and JAK3 was developed with DAB.
Results
Histology for apoptosis and mitosis
To assess any temporal association between altered JAK3 mRNA (Table 1) and apoptosis or mitosis, percentage change in these parameters was investigated in control, SF and 1.0 mM H2O2-treated cultures of NRK49F fibroblast and NRF52E tubular epithelial cells at 24 h of treatment. Figure 1 demonstrates percentage change in apoptosis (A) and mitosis (B). In Figure 1A, the low levels of apoptosis in both cell lines for control or SF medium were significantly increased compared with oxidative stress (1.0 mM H2O2). The increase was 10-fold greater in fibroblasts and fivefold greater in epithelial cells compared with controls. In Figure 1B, levels of mitosis seen in control and SF cultures were significantly decreased (P < 0.05) compared with 1.0 mM H2O2. Mitosis was almost negated in NRK52E cells with oxidative stress. Temporally, these changes in apoptosis and mitosis with oxidative stress were associated with the decrease in JAK3 mRNA in both cell lines.
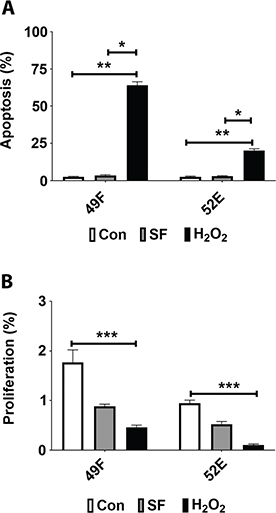
Figure 1. Apoptosis and mitosis in kidney fibroblast and tubular epithelial cells 24 h after oxidative stress. To assess any temporal association between altered JAK3 mRNA and apoptosis or mitosis, percentage change in these parameters was investigated in parallel control, serum-free (SF) and 1.0 mM H2O2-treated cultures of normal rat kidney fibroblast cells (NRK49F; 49F) and normal rat kidney tubular epithelial cells (NRF52E; 52E). In (A), the low levels of apoptosis in both cell lines for control and SF medium were significantly increased with 1.0 mM H2O2. In (B), levels of mitosis seen in control and SF cultures were significantly decreased with 1.0 mM H2O2. *P < 0.05; **P < 0.01 and ***P < 0.001.
Western blot analysis of JAK3 protein in in vitro and in vivo experiments
To determine if the changes in mRNA in JAK3 were translated to the protein, extracts from control, SF and 1.0 mM H2O2-treated cultures were subjected to Western blotting. Figure 2A demonstrates JAK3 protein expression in cell culture experiments, for NRK49F and NRK52E cells for control, SF medium and medium containing 1.0 mM H2O2 for 18–20 h. These were the same experiments used for the mRNA analyses (Table 1) and for histological analysis of apoptosis and mitosis (Figure 1). JAK3 protein expression (125 kDa approximately) was not significantly altered from controls after H2O2-treatment in either cell line, although JAK3 expression for NRK52E in the SF treatment tended to be lower than the normal medium controls, and the H2O2 treatment (Figure 2A). To determine if JAK3 protein expression was altered in vivo, Western immunoblotting was performed on protein extracts from whole kidney tissue taken from UUO rats at 0 (control), 6 h, 1, 2, 4 and 7 days. Significant levels of oxidative stress and tubulointerstitial fibrosis had been reported by us previously at 4 days of obstruction (12). Figure 2B demonstrates that there was no alteration in whole kidney JAK3 protein from controls in UUO kidneys from animals with increasing duration of UUO (6 h, 24 h, 2, 4 and 7 days).
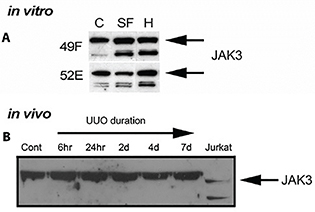
Figure 2. Western immunoblots of JAK3 protein from in vitro and in vivo experiments. In (A), to determine qualitatively if the changes in RNA in JAK3 were translated to protein expression, extracts from control, serum-free (SF) and 1.0 mM H2O2 (H)-treated cultures were subjected to Western blotting for NRK49F (49F) and NRK52E (52E) cells. JAK3 protein expression (band size 125 kDa approximately) was not altered from controls after SF and H2O2-treatment in 49F cells but a small increase was detected after H2O2 treatment in 52E cells. The stable or increased protein expression was different from mRNA levels which were several fold lower than controls. In (B), to determine if JAK3 protein expression was altered in vivo, Western immunoblotting was performed on tissue taken from UUO rats at 0 (control), 6 h and 24 h, and 2, 4 and 7 days. There was no alteration in whole kidney JAK3 protein from controls in UUO kidneys from animals with increasing duration of UUO (6 h, 24 h, 2, 4 and 7 days). Jurkat cells were used as positive control.
Phosphorylation of JAK3 and association with phosphorylated STAT3
The differences between mRNA and protein from in vitro Western blot results of JAK3 suggested post-transcriptional control on JAK3. To investigate if JAK3 activation (phosphorylation) was altered, pTyr of JAK3 and phosphorylated STAT3 (pSTAT3) were analysed (Figure 3). In Figure 3A, similar to JAK3 protein expression (Figure 2A), pTyr of JAK3 in tubular epithelial NRK52E cells was significantly increased after oxidative stress. In NRK49F cells, although JAK protein expression was stable (Figure 2A), JAK3 activation (p-Tyr) decreased with oxidative stress (Figure 3B), thereby correlating with the microarray results of decreased JAK3 mRNA for these cells. In Figure 3C, pSTAT3 expression was investigated in an attempt to determine its post-transcriptional association with JAK3. STAT3 phosphorylation was negligible in both H2O2-treated cell lines. The SF controls for the NRK49F cells also had negligible pSTAT3. The pSTAT3 results were counterintuitive (pSTAT3 often increased in fibrosis) but may be the result of loss of pSTAT3-stimulatory cytokines after SF and oxidative stress treatments. This requires further study.
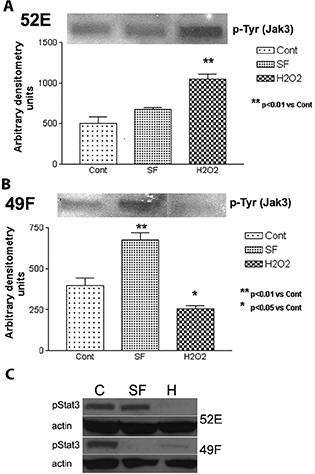
Figure 3. Western blots showing phosphorylation of JAK3 and STAT3. In (A) and (B), phospho-tyrosine (pTyr) of JAK3 in tubular epithelial NRK52E (52E) and NRK49F (49F) cells was opposite in expression patterns after oxidative stress, with an increase in 52E and a decrease in 49F. In (C), phosphorylated STAT3 (pStat3) expression was negligible in both H2O2-treated cell lines and the SF controls for the NRK49F cells.
Localisation of JAK3 during UUO
To determine whether localisation of JAK3 altered during the development of kidney fibrosis during UUO (proven association with oxidative stress) from that seen in control kidneys, IHC analysis was conducted on paraffin-embedded sections using the same antibody to JAK3 as was used for Western immunoblots. Activation of JAK3 was not investigated. Figure 4A shows negligible JAK3 in the cortex of control kidneys. Figure 4B demonstrates localisation of JAK3 to vascular endothelium (red blood cells visible in the vascular lumens), but little expression otherwise, in the outer stripe of the medulla of controls. With increasing duration of UUO, there was positive JAK3 staining of tubular epithelial cells in the cortical regions (4C, 7 days UUO demonstrated). In all cases, subcellular localisation of JAK3 was cytoplasmic. Tubular epithelium with apoptotic cells had little to no JAK3 expression. There were very few interstitial cells (fibroblasts) that stained positive for JAK3.
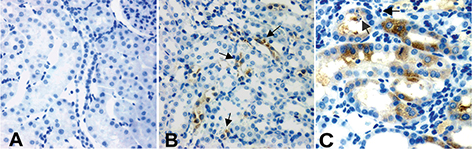
Figure 4. Localisation of JAK3 during UUO. In all cases, subcellular localisation of JAK3 was cytoplasmic; (A) shows negligible JAK3 in the cortex of control kidneys; (B) demonstrates localisation of JAK3 to vessels (arrows), but little expression otherwise, in the medulla of controls. With increasing duration of UUO, there was positive JAK3 staining of tubular epithelial cells in the cortical regions (4C, 7 days UUO demonstrated). In general, apoptotic cells did not show JAK3 localisation and tubular epithelium with apoptotic cells had little to no JAK3 expression (arrows). The most intense staining was observed over mitotic epithelium (C). There were very few interstitial cells (fibroblasts) that stained positive for JAK3.
Localisation of JAK3 with pERK in vivo during UUO
Double IHC staining confirmed that JAK3 was mainly localised to tubular cells at the later stages of UUO (4–7 days UUO), but not associated with apoptotic epithelium in UUO. It is possible that JAK3 expression was in apoptotic cells of the epithelium and that the shrinkage of apoptotic cells during the death process meant the JAK3 positivity was not detected. This would influence the lack of co-localisation of JAK3 and pERK (Figure 5), with one interpretation being that JAK3 did not localise to apoptotic cells that occur in this structure (12) and the other interpretation being that the apoptotic cell structure precluded detection of JAK3 in the cytoplasm.
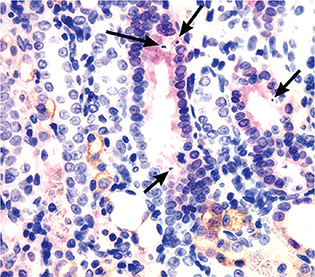
Figure 5. Localisation of JAK3 with phosphorylated ERK (pERK) in the cortex during UUO. Double IHC staining confirmed JAK3 (brown staining) was mainly localised to tubular epithelial cells (7 days UUO demonstrated), but not associated with apoptotic cells (examples arrowed). Rather, apoptosis was localised to epithelium expressing pERK (red stain). JAK3 and pERK did not co-localise.
Discussion
We had demonstrated previously, using an in vitro model of oxidative stress-induced renal fibrosis, that activated or phosphorylated extracellular signal-regulated protein kinase (pERK) played a role in apoptosis of renal fibroblasts, but not tubular epithelium where it promoted cell growth and survival (11). Using the UUO model, we then found that, during the fibrotic process, patchy promotion of tubular epithelial proliferation and survival, and interstitial cell apoptosis were linked spatially and temporally with increased pERK expression (12). There is evidence of an interactive mechanistic pathway between the MAPK signalling pathway and JAK/STAT signalling (18, 19), and for a role for the JAK/STAT pathway in kidney fibrosis (3–6). In this study, where we investigated mRNA for JAK1, JAK2 and JAK3, only JAK3 mRNA had any marked change using the established in vitro model with cells central to development of fibrosis (tubular epithelium and renal fibroblasts). The multifold change in expression was downregulation of JAK3 mRNA. JAK3 is a known tyrosine kinase pathway signalling molecule. Our hypothesis was that, if JAK3 mRNA was decreased in association with renal fibrogenesis, then JAK3 protein expression would also be decreased. In contrast, we found that JAK3 protein levels in the cell culture oxidative stress experiments, experiments identical to the mRNA analysis, were unaltered (NRK49F) or increased (NRK52E), indicating possible post-transcriptional regulation of JAK3. This report attempts to explain the disparate results found between mRNA and protein analyses.
The causes and molecular mechanisms mediating kidney fibrosis, and the diseases such as chronic kidney disease or diabetic nephropathy in which fibrosis plays a central role, are complex. Regulation and/or dysregulation of numerous biological processes, including oxidative stress, remodelling of cellular function and morphology, and disturbance of metabolic pathways, are all key mechanisms. The intricate network of transcriptional, post-transcriptional and translational processes is known to act throughout biology to determine functional outcomes (20). Among these, mechanisms for the regulation of protein translation are not well understood. In our models, mRNA downregulation of JAK3 was not replicated at protein levels in the same experimental conditions that produced evidence of JAK3 mRNA downregulation. To investigate if JAK3 activation was altered, phosphorylation of tyrosine kinase (pTyr) of JAK3 protein was analysed. pTyr of JAK3 was significantly decreased by oxidative stress treatment versus control treatment in the kidney fibroblast NRK49F cells, thereby correlating with the microarray results. However, pTyr of JAK3 in tubular epithelial NRK52E cells was significantly increased after oxidative stress. Thus, although JAK3 protein expression was stable or increased after oxidative stress, phosphorylation of JAK3 did not explain these differences between mRNA and protein in fibroblast and tubular epithelial cells.
A possible explanation could be that the JAK/STAT pathway interaction determined outcome. The STATs are tyrosine phosphorylated by JAKs in the cytoplasm and then proceed to translocate into the nucleus where they act as transcription factors (2). Liu et al. (18) have demonstrated that STAT3 and JAK3 may act together, with JAK activation determining STAT activation, when investigating therapeutic effects of some cytokine inhibitors in diabetic nephropathy. There is other evidence that the JAK/STAT pathways act in diabetic nephropathy and may provide novel targets for new therapies (21–23). In this study, activated STAT3 (pSTAT3) expression was negligible in fibroblast and epithelial cell cultures treated with H2O2 and so again little explanation was found for the discrepancies in JAK3 mRNA and protein after oxidative stress. In the in vitro experiments, these changes were linked temporally with increased apoptosis and decreased cell proliferation during oxidative stress, in both cell types. At least in kidney epithelial cells in the cell culture model, JAK3 activation may promote fibrosis via tubular atrophy. The links with increased fibroblast apoptosis would indicate a role in modulating fibrosis. Koike et al. (4) reported that JAK/STAT pathway activation ameliorated kidney fibrosis, using the in vivo UUO model of kidney fibrosis.
Information regarding the JAK/STAT pathway and apoptosis are, in general, conflicting. Induction of apoptosis in cancer cells has been associated with the induction of the JAK/STAT signalling pathway after death receptor activation (24). In contrast, activation of neuronal erythropoietin receptors prevented apoptosis induced by N-methyl-d-aspartate or nitric oxide by triggering cross-talk between the signalling pathways of JAK2 and nuclear factor-kappaB (25). The true role of JAK3 in renal fibrosis needs further study, perhaps using specific inhibitors of JAK3.
Although Western immunoblot indicated minimal change in the protein levels of JAK3 protein in vitro and no change in vivo, a comparison was made using IHC analysis of kidney fibrosis that developed using the UUO model of tubulointerstitial fibrosis (12). This investigation indicated altered localisation, particularly in the later stages of UUO, where expression of JAK3 was increased in cortical tubular epithelial cells (but not in overall in the whole kidney). It is this nephron segment that undergoes significant atrophy, involving apoptosis, but also regeneration, involving tubular epithelial cell proliferation (26). JAK3 localisation in various parts of the kidney may be more important than total kidney expression. In addition, use of a phosphorylated JAK3 antibody for the IHC analysis may have produced different results and needs further study.
We have previously shown a role for the MAPK pathway, in particular ERK, in renal tubulointerstitial fibrosis in cell culture and during UUO (11, 12). Han and colleagues have also demonstrated a similar outcome, although they found activated ERK (pERK) localised to interstitial macrophages in the UUO model (13). The MAPK pathway of signal transduction molecules often acts in parallel or in synergy with other signalling pathways. Identification of alternative or complementary pathways to MAPK may help define their role in renal fibrogenesis; however in our UUO study, JAK3 protein was not found to co-localise with pERK. JAK3 appeared to play a role in tubular cell survival or regeneration during fibrogenesis, as it was seen in association with mitotic cells in cortical tubular epithelium in UUO. There did not appear to be direct “cross-talk” between pERK and JAK3 as there was no co-localisation of these proteins. The present results do, however, give some indication of the independent functioning of pERK and JAK3 in renal fibrosis.
In conclusion, the gene profiling of renal tubular epithelial and fibroblast cells under oxidative stress indicated significant decreases in JAK3 gene expression that was not mirrored by altered protein expression in the same cells. Activation of JAK3 may be a key to some of the cellular changes known to occur in kidney fibrosis. This study undoubtedly leaves many questions unanswered but does indicate that multiple signalling pathways are important in oxidative injury and kidney fibrogenesis, and it may be more important to understand their interrelationships rather than focusing on the role of a single independent pathway. The disparate results for JAK3 mRNA and protein expression mean that this study cannot make a case for or against JAK3 having a role in renal fibrosis.
Acknowledgements
Dr Glen Boyle, QIMR-Berghoffer Research Institute, Brisbane, Australia, and Dr Helen Healy, Department of Nephrology, Royal Brisbane and Women’s Hospital, Brisbane, Australia, are acknowledged for advice and consultation for some parts of this project.
Conflict of interest statement
The authors report no conflict of interest with respect to research, authorship and/or publication of this article.
References
- Chuang PY, He JC. JAK/STAT signaling in renal diseases. Kidney Int. 2010 Aug;78(3):231–4. http://dx.doi.org/10.1038/ki.2010.158
- Matsui F, Meldrum KK. The role of the Janus kinase family/signal transducer and activator of transcription signaling pathway in fibrotic renal disease. J Surg Res. 2012 Nov;178(1):339–45. http://dx.doi.org/10.1016/j.jss.2012.06.050
- Guh JY, Huang JS, Chen HC, Hung WC, Lai YH, Chuang LY. Advanced glycation end product-induced proliferation in NRK-49F cells is dependent on the JAK2/STAT5 pathway and cyclin D1. Am J Kidney Dis. 2001 Nov;38(5):1096–104. http://dx.doi.org/10.1053/ajkd.2001.28616
- Koike K, Ueda S, Yamagishi S, Yasukawa H, Kaida Y, Yokoro M, et al. Protective role of JAK/STAT signaling against renal fibrosis in mice with unilateral ureteral obstruction. Clin Immunol. 2014 Jan;150(1):78–87. http://dx.doi.org/10.1016/j.clim.2013.11.003
- Huang JS, Guh JY, Hung WC, Yang ML, Lai YH, Chen HC, et al. Role of the Janus kinase (JAK)/signal transducters and activators of transcription (STAT) cascade in advanced glycation end-product-induced cellular mitogenesis in NRK-49F cells. Biochem J. 1999 Aug 15;342 (Pt 1):231–8. http://dx.doi.org/10.1042/bj3420231
- Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81(1):102–13. http://dx.doi.org/10.1002/1097-4644(20010401)81:1%3C102::AID-JCB1027%3E3.0.CO;2-Y
- Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010 Aug;78(3):257–68. http://dx.doi.org/10.1038/ki.2010.154
- Horiguchi A, Oya M, Marumo K, Murai M. STAT3, but not ERKs, mediates the IL-6-induced proliferation of renal cancer cells, ACHN and 769P. Kidney Int. 2002 Mar;61(3):926–38. http://dx.doi.org/10.1046/j.1523-1755.2002.00206.x
- Dai T, Wang Y, Nayak A, Nast CC, Quang L, LaPage J, et al. Janus kinase signaling activation mediates peritoneal inflammation and injury in vitro and in vivo in response to dialysate. Kidney Int. 2014 Dec;86(6):1187–96. http://dx.doi.org/10.1038/ki.2014.209
- Wiezel D, Assadi MH, Landau D, Troib A, Kachko L, Rabkin R, et al. Impaired renal growth hormone JAK/STAT5 signaling in chronic kidney disease. Nephrol Dial Transplant. 2014 Apr;29(4):791–9. http://dx.doi.org/10.1093/ndt/gfu003
- Pat BK, Cuttle L, Watters D, Yang T, Johnson DW, Gobe GC. Fibrogenic stresses activate different mitogen-activated protein kinase pathways in renal epithelial, endothelial or fibroblast cell populations. Nephrology (Carlton). 2003 Aug;8(4):196–204. http://dx.doi.org/10.1046/j.1440-1797.2003.00162.x
- Pat B, Yang T, Kong C, Watters D, Johnson DW, Gobe G. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: Modulation by antioxidants. Kidney Int. 2005 Mar;67(3):931–43. http://dx.doi.org/10.1111/j.1523-1755.2005.00157.x
- Han Y, Masaki T, Hurst LA, Ikezumi Y, Trzaskos JM, Atkins RC, et al. Extracellular signal-regulated kinase-dependent interstitial macrophage proliferation in the obstructed mouse kidney. Nephrology (Carlton). 2008 Oct;13(5):411–18. http://dx.doi.org/10.1111/j.1440-1797.2008.00926.x
- Percy CJ, Pat BK, Healy H, Johnson DW, Gobe GC. Phosphorylation of caveolin-1 is anti-apoptotic and promotes cell attachment during oxidative stress of kidney cells. Pathology. 2008 Dec;40(7):694–701. http://dx.doi.org/10.1080/00313020802436402
- Gobe G. Identification of apoptosis in kidney tissue sections. Methods Mol Biol. 2009;466:175–92. http://dx.doi.org/10.1007/978-1-59745-352-3_13
- Davidson K, Percy C, Rennick AJ, Pat BK, Li J, Nicol D, et al. Comparative analysis of caspase activation and apoptosis in renal tubular epithelial cells and renal cell carcinomas. Nephron Exp Nephrol. 2005;99(4):e112–20. http://dx.doi.org/10.1159/000083926
- Rajandram R, Yap NY, Pailoor J, Razack AH, Ng KL, Ong TA, et al. Tumour necrosis factor receptor-associated factor-1 (TRAF-1) expression is increased in renal cell carcinoma patient serum but decreased in cancer tissue compared with normal: Potential biomarker significance. Pathology. 2014 Oct;46(6):518–22. http://dx.doi.org/10.1097/PAT.0000000000000145
- Liu Q, Xing L, Wang L, Yao F, Liu S, Hao J, et al. Therapeutic effects of suppressors of cytokine signaling in diabetic nephropathy. J Histochem Cytochem. 2014 Feb;62(2):119–28.
- Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur J Pharmacol. 2016 Nov 15;791:8–24. http://dx.doi.org/10.1016/j.ejphar.2016.08.022
- Viengchareun S, Lema I, Lamribet K, Keo V, Blanchard A, Cherradi N, et al. Hypertonicity compromises renal mineralocorticoid receptor signaling through Tis11b-mediated post-transcriptional control. J Am Soc Nephrol. 2014 Oct;25(10):2213–21. http://dx.doi.org/10.1681/ASN.2013091023
- Chen SC, Guh JY, Chen HC, Yang YL, Huang JS, Chuang LY. Advanced glycation end-product-induced mitogenesis is dependent on Janus kinase 2-induced heat shock protein 70 in normal rat kidney interstitial fibroblast cells. Transl Res. 2007 May;149(5):274–81. http://dx.doi.org/10.1016/j.trsl.2006.08.005
- Brosius FC, Khoury CC, Buller CL, Chen S. Abnormalities in signaling pathways in diabetic nephropathy. Expert Rev Endocrinol Metab. 2010;5(1):51–64. http://dx.doi.org/10.1586/eem.09.70
- Khan SS, Quaggin SE. Therapies on the horizon for diabetic kidney disease. Curr Diab Rep. 2015 Dec;15(12):111. http://dx.doi.org/10.1007/s11892-015-0685-3
- Choi EA, Lei H, Maron DJ, Wilson JM, Barsoum J, Fraker DL, et al. Stat1-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand and the cell-surface death signaling pathway by interferon beta in human cancer cells. Cancer Res. 2003 Sep 1;63(17):5299–307.
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001 Aug 9;412(6847):641–7. http://dx.doi.org/10.1038/35088074
- Peake JM, Gobe GC, Fassett RG, Coombes JS. The effects of dietary fish oil on inflammation, fibrosis and oxidative stress associated with obstructive renal injury in rats. Mol Nutr Food Res. 2011 Mar;55(3):400–10. http://dx.doi.org/10.1002/mnfr.201000195