CASE REPORT
Monitoring the Effects of Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide Switch for Tubulotoxicity in Highly Treatment-Experienced or in Very Sick Individuals Infected with HIV
Nicole Lioufas1, Alan Street2, Paul Champion de Crespigny1, Stephen G. Holt1,3
1Department of Nephrology, The Royal Melbourne Hospital, Parkville, Australia; 2Victorian Infectious Diseases Service, The Royal Melbourne Hospital, Parkville, Australia; 3School of Medicine, The University of Melbourne, Parkville, Australia
Abstract
Tenofovir disoproxil fumarate (TDF) is a common antiretroviral utilised in the treatment of human immunodeficiency virus (HIV) and hepatitis B infections. It is associated with the development of tubulotoxicity and tubulopathies, and is not recommended in the treatment of patients with baseline chronic kidney disease. Until now, guidelines have suggested frequent monitoring of serum biochemistry to detect the development of such complications. In recent trials, a new prodrug formulation of tenofovir alafenamide (TAF) has been shown to exhibit less tubular toxicity than its counterpart due to a lower serum concentration of its metabolites. In this article, we share our experience with two patients who developed tubulotoxicity following the commencement of TDF-based regimens in HIV, and its improvement following its change to TAF, and review the available literature regarding tenofovir-based nephrotoxicity.
Keywords: anti-retroviral; HIV; tenofovir alafenamide; tenofovir disoproxil fumarate; tubulotoxicity;
Received: 27 April 2018;
Accepted after revision: 11 June 2018;
Published: 29 June 2018
Author for correspondence: Nicole Lioufas, Department of Nephrology, Royal Melbourne Hospital, Parkville, Australia. Email:
Nicole.Lioufas2@wh.org.auHow to cite: Lioufas N et al. Monitoring the effects of tenofovir disoproxil fumarate to tenofovir alafenamide switch for tubulotoxicity in highly treatment-experienced or in very sick individuals infected with HIV. J Ren Hepat Disord. 2018;2(2):1–5.
Doi:
http://dx.doi.org/10.15586/jrenhep.2018.33Copyright: Lioufas N et al.
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
Combined anti-retroviral treatment (cART) regimens for human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infection are often formulated for patients with normal renal function, and finding suitable regimes for patients whose renal function has deteriorated can be challenging. Tenofovir disoproxil fumarate (TDF), a nucleotide reverse transcriptase inhibitor (NRTI), has been extensively used as a ‘backbone’ for such treatment, with more than 50% of patients on a TDF-based regimen. TDF is a prodrug, being converted intracellularly to tenofovir diphosphate, a structural analogue of deoxyadenosine triphosphate which suppresses viral replication by inhibiting viral reverse transcriptase (1). However, TDF has been associated with the development of a progressive, predominantly proximal, tubulopathy with renal impairment in a small number of patients (2). Frequent renal function testing has been advocated to detect this complication (3), and proximal tubulopathy may be detected by looking for evidence of metabolic acidosis, hypophosphatemia, hyperphosphaturia, hypokalaemia, hyperuricaemia, tubular proteinuria, aminoaciduria and glycosuria. Urinary tubular markers are expensive and difficult to monitor; therefore, tubular proteinuria may be inferred by the ratio of urinary albumin (conveniently measured by urine albumin to creatinine ratio (uACR) on a spot sample) compared with urinary total protein (measured with urine protein to creatinine ratio [uPCR]) (4). Thus, uACR/uPCR is a simple way to monitor tubular dysfunction in patients with HIV, with a urine albumin to protein ratio (uAPR) of ~0.4 or less suggesting tubular proteinuria (5). However, for patients with creatinine clearance <50 ml/min, TDF is not recommended by prescribing guidelines, and if used should be dose reduced. However, finding a suitable regimen to switch to in such patients may be tricky due to the side effects of alternative medications or preexisting antiretroviral resistance. Tenofovir alafenamide (TAF), another prodrug of tenofovir, which is preferentially concentrated in lymphoid tissue, offers an improved renal safety profile and may offer a simple switch in patients with renal issues, where alternatives may be less desirable. We report the cases of two complex patients in whom TDF-based therapy was associated with significant side effects, who showed clear benefit by a switch from TDF to TAF.
Case 1
A 62-year-old man diagnosed with HIV-1 infection in 1985 was referred in 2013 for assessment of renal impairment and proteinuria. He was highly treatment experienced and previous genotypic testing had demonstrated acquired HIV resistance to all NRTIs except for intermediate resistance to emtricitabine, to all non-nucleoside RTIs, to all protease inhibitors except low level resistance to darunavir, and likely non-response to the entry inhibitor maraviroc. Nevertheless, his treatment regimen of TDF, emtricitabine, ritonavir-boosted darunavir and raltegravir kept his HIV viral load undetectable and CD4 count within the reference range (480 cells/μL [reference range 400–1500]). Other comorbidities included previous Hodgkin’s lymphoma diagnosed and treated in 2007 with adriamycin, bleomycin, vincristine and dacarbazine from which he achieved a complete response, with no obvious immediately or long-term toxicities noted. He had type-2 diabetes mellitus for 8 years treated with oral hypoglycaemic agents, cirrhosis due to non-alcoholic steatohepatitis, hyperlipidaemia on a statin, paroxysmal atrial flutter and treated hypertension. He had no family history of renal disease, did not consume alcohol and had given up smoking more than 10 years ago.
His creatinine had been stable at ~110 μmol/L (MDRD ~58 eGFR ml/min/1.73 m2) for the last 3 years, but at presentation his urinary protein level had begun to creep up with a urine protein–creatinine ratio of 110 mg/mmol and an albumin–creatinine ratio of 40 mg/mmol. At clinic visits prior to this, his blood pressure had been well controlled (<140/80 mmHg). Most electrolytes were within reference ranges, but serum bicarbonate 21 mmol/L (25–35 mmol/L), corrected calcium 2.39 mmol/L (2.1–2.6 mmol/L), magnesium 0.84 mmol/L (0.7–1.1) and phosphate 0.32–0.80 mmol/L (0.80–1.5). At this time, he was also noted to be glycosuric on dipstick with normal plasma glucose. Ultrasound showed normal renal sized kidneys and his autoantibodies were negative. There was no change in urinary protein leak in response to the addition of an angiotensin-converting enzyme inhibitor.
Given this patient’s previous treatment exposure, resistance mutations and comorbidities, the options for alternative treatments were extremely limited. Initially, a trial of dose reduction of the TDF component of his therapy was attempted, reducing 300 mg daily to 300 mg second daily dosing. However, there was no significant improvement in renal parameters including those of glomerular filtration rate (GFR), proteinuria, serum bicarbonate, or phosphate levels, but his HIV viral load remained suppressed on a lower dose regimen.
A switch from TDF to TAF 10 mg daily was performed, and he remained on emtricitabine 200 mg daily. On a TAF-based regimen, there were significant improvements within a month (mean ± standard deviation) in uPCR 72 ± 26 to 56 ± 17 mg/mmol (p < 0.05), uAPR 0.3 ± 0.06 to 0.39 ± 0.07, (P < 0.001), serum albumin 42 ± 3 to 44 ± 4 (P < 0.05), serum phosphate (0.69 ± 0.16 to 0.87 ± 0.13 mmol/L (P < 0.001) and serum urate (0.21 ± 0.01 to 0.26 ± 0.03; p < 0.02) (Figure 1). Serum creatinine (112 ± 8 to 115 ± 10, p = ns), uACR (23 ± 10 to 23 ± 10, p = ns) and eGFR (58 ± 5 to 55 ± 5, p = ns) remained unchanged and viral load stayed undetectable with good CD4 counts.
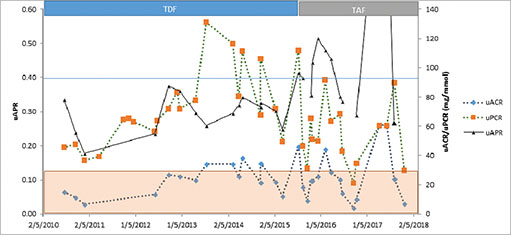
Figure 1. This graph illustrates the reduction in proteinuria (uPCR), initially without much change in albuminuria (uACR) after starting TAF. This increases uAPR above 0.4 for the first time since the patient developed proteinuria. The significance of uAPR with low total urine protein is unknown and is not shown when uPCR < 30 mg/mmol shaded (as per Ref [5]).
Case 2
A 45-year-old man with a history of amphetamine use was admitted with exertional dyspnoea, 15 kg weight loss and intermittent fever, but no cough or orthopnoea. Chest X-Ray showed bilateral pulmonary infiltrates and follow-up computed tomography of the chest showed bilateral central ground glass changes with associated interstitial septal thickening and abruptly demarcated upper lobe emphysema. An HIV test was positive, HIV viral load was 982, 444 copies/ml and CD4 count was 20 cells/μL. Pneumocystis jiroveci pneumonia (PJP) was confirmed at bronchoscopy in addition to Myocbacterium avium complex (MAC). He was treated with high-dose trimethoprim/sulphamethoxazole, prednisolone, ethambutol, clarithromycin and rifampicin.
At admission, his creatinine was 75 μmol/L, and no proteinuria was noted upon urine dipstick. His admission was further complicated by staphylococcal pneumonia, and cytomegalovirus encephalitis treated with valganciclovir. His creatinine peaked at 130 μmol/L, which then normalised to 70 μmol/L as he recovered from his acute infection. Two weeks after admission he was started on TDF/emtricitabine. Within 1 week, his serum phosphate fell to a nadir of 0.31 mmol/L and he required significant phosphate replacement, up to 100 mmol/day, in addition to diet (recommended dietary intake 32 mmol/day) for 2 weeks, with inability to wean. Fractional excretion of phosphate was 42% (normal < 20%). Additionally, he had a protein–creatinine ratio of 179 mg/mmol, and an urine ACR of 9.29 mg/mmol (uAPR of 0.05). He was switched to TAF/emtricitabine and improvement was noted with his electrolytes within 1 week, with a rapid wean of his phosphate replacement until stabilisation of serum phosphate at 1.2 mmol/L within 1 week (Figure 2). One month following his change to TAF, his creatinine remained within the normal range and his levels of proteinuria fell to within the normal range by 3 months (PCR 28 mg/mmol) and by 1 year (PCR 18, ACR 1.1 mg/mmol). Phosphate levels have remained within the normal range on no replacement.
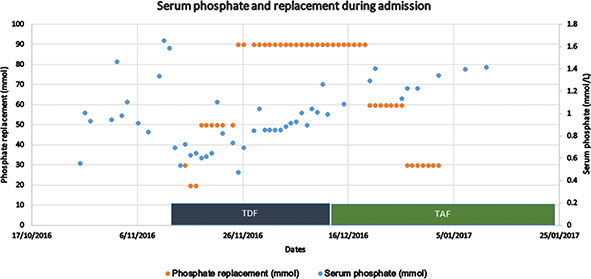
Figure 2. Serum phosphate levels rise and oral phosphate replacement requirements fall significantly and acutely on change from TDF to TAF.
Discussion
An estimated 36.7 million people were living with HIV in 2016 (6) and the increasing prevalence is mainly related to the longer life expectancy due to cART (7). Thus, an older prevalent population is emerging with many years of well-suppressed HIV who are developing more issues related to chronic disease such as cardiovascular disease, diabetes and chronic kidney disease (CKD). The complex interplay between chronic viral infection, cART and natural ageing contributes to increased chronic morbidity in this population (8). Patients living with HIV have higher rates of CKD than the general population, in developed countries, and whilst specific HIV-related glomerular disorders (e.g. HIV-associated nephropathy or immune complex kidney disease) contribute to renal disease burden, this is becoming an increasingly rare contributor to the causes of kidney dysfunction (9). Tubulointerstitial disorders and tubulopathies are estimated to cause approximately 15% of renal disease in retrospective renal biopsy series (10–12). In a retrospective cohort single-centre study, 222 renal biopsies from HIV-positive patients were analysed for aetiology of renal impairment. One-third of biopsies were thought to show tubulopathy, of which ~80% were thought to be drug related and TDF was the main culprit. A further half of the biopsies showed acute or chronic interstitial nephritis, and TDF was implicated in approximately one quarter of these (12). It should be noted that interstitial nephritis in this biopsy series was also associated with opportunistic infections such as MAC or tuberculosis.
Current recommendations for TDF-related nephrotoxicity are vague, given the absence of strong evidence. In 2014, the Infectious Diseases Society of America (IDSA) guidelines recommended that all patients should be screened regularly for blood pressure, CKD and electrolyte parameters at diagnosis and at the commencement of TDF-based therapy (13). In the more recent Australian consensus statement, it was recommended that both the urinary albumin and urinary protein should be measured separately to detect the possible development of tubular proteinuria (3). Furthermore, IDSA recommends that regimens containing TDF should not be commenced if the patient has a GFR < 60 ml/min, and to change antiretroviral therapy from TDF-containing regimens if there is a >25% decline in GFR or development of tubular dysfunction (13). Similar recommendations have been made by the European Association for the Study of the Liver (14).
The pharmacokinetics of TAF make this an ideal replacement for TDF as similar intracellular levels in target cells allow much lower levels of plasma tenofovir, and hopefully lower incidence of nephrotoxicity. Randomised studies of TAF- versus TDF-based regimes in patients with creatinine clearance >50 ml/min showed non-inferior efficacy in viral suppression and a smaller increase in creatinine and lower increases in urinary protein at 48 weeks (15). One single-arm open-label study switching patients with renal dysfunction (estimated creatinine clearance 30–60 ml/min) from TDF- to TAF-based regimes showed improvement in proteinuria (16). A more recent randomised control trial of TDF to TAF in 1443 HIV-1 patients revealed non-inferiority in terms of viral suppression at 96 weeks, with improvement in GFR and bone mineral density (17). Similar evidence in the treatment of Hepatitis B has emerged with again non-inferiority and improved renal outcomes in phase 3 trials (18, 19). In these cases, we observed a change in tubular proteinuria following the change from TDF to TAF. Case 1 was noted at referral to have a creatinine of approximately 110 μmol/L, which did not improve significantly following his change in drug regimen. This patient had other risk factors for preexisting renovascular CKD, which would likely account for no change in creatinine despite change to TAF. The improvement in electrolyte parameters and tubular proteinuria in both patients suggests that there is a degree of reversibility, which is likely to be more significant if the earlier change in therapy from TDF to TAF is made.
The World Health Organization guidelines regarding antiretroviral therapy mention caution with regard to CKD and suggest that TAF may be helpful in this patient group; however, the guidelines have not routinely recommended the drug due to lack of safety data in pregnant women (20, 21). TAF has been more recently approved in the USA, Europe (22) and recently in Australia as part of fixed-dose formulations in combination with emtricitabine alone or with addition of a third antiretroviral agent.
In summary, we show here that it appears safe and efficacious to switch treatment-experienced and inexperienced patients with tubulopathy and renal impairment to a TAF-based regimen. The efficacy of such a switch can be assured by following biochemical markers suggestive of tubular damage like proteinuria, uAPR and the degree of phosphaturia.
Conflict of Interest
Stephen G. Holt has received research funding and/or honoraria from Amgen, Astra Zenica, Baxter, Gilead and Sanofi. The other authors declare no potential conflicts of interest with respect to research, authorship and/or publication of this article.
References
References
- Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Nino MD, Izquierdo MC, Ppveda J, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908.
- Gupta SK, Anderson AM, Ebrahimi R, Fralich T, Graham H, Scharen-Guivel V, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: A prospective, case-control study of predictors and resolution in HIV-infected patients. PLoS One. 2014;9(3):e92717. http://dx.doi.org/10.1371/journal.pone.0092717
- Holt SG, Gracey DM, Levy MT, Mudge DW, Irish AB, Walker RG, et al. A consensus statement on the renal monitoring of Australian patients receiving tenofovir based antiviral therapy for HIV/HBV infection. AIDS Res Treat. 2014;10(11):35.
- Smith ER, Cai MM, McMahon LP, Wright DA, Holt SG. The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients. Nephrol Dial Transplant. 2012;27(4):1534–41. http://dx.doi.org/10.1093/ndt/gfr708
- Samarawickrama A, Cai M, Smith E, Nambiar K, Sabin C, Fisher M, et al. Utility of uAPR measurement in HIV infection. HIV Med. 2012;13:526–32. http://dx.doi.org/10.1111/j.1468-1293.2012.01003.x
- World Health Organisation. Progress report 2016: Prevent HIV, test all and treat all. Geneva: World Health Organisation; 2016.
- Maartens G, Celum C, Lewin SR. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258–71. http://dx.doi.org/10.1016/S0140-6736(14)60164-1
- Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus. NEJM. 2003;348:702–10. http://dx.doi.org/10.1056/NEJMoa022048
- Rao TKS, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310(11):669–73. http://dx.doi.org/10.1056/NEJM198403153101101
- Berliner A, Fine D, Lucas G, Rahman M, Racussen L, Scheel P, et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28(3):478–86. http://dx.doi.org/10.1159/000112851
- Wyatt CM, Morgello S, Katz-Malamed R, Wei C, Klotman ME, Klotman PE, et al. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2009;75(4):428–34. http://dx.doi.org/10.1038/ki.2008.604
- Zaidan M, Lescure FX, Brocherou I, Dettwiler S, Guiard-Schmid JB, Pacanowski J, et al. Tubulointerstitial nephropathies in HIV-infected patients over the past 15 years: A clinico-pathological study. Clin J Am So Nephrol. 2013;8:930–8. http://dx.doi.org/10.2215/CJN.10051012
- Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(9):e96–e138. http://dx.doi.org/10.1093/cid/ciu730
- European Association For the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. http://dx.doi.org/10.1016/j.jhep.2012.02.010
- Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–16. http://dx.doi.org/10.1016/S0140-6736(15)60616-X
- Pozniak A, Arribas JR, Gathe J, Gupta SK, Post FA, Bloch M, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. Acquir Immune Defic Syndr. 2016;71:530–7. http://dx.doi.org/10.1097/QAI.0000000000000908
- Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: A randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52. http://dx.doi.org/10.1016/S1473-3099(15)00348-5
- Chan HLY, Fung S, Seto WK, Chuang W-L, Chen C-Y, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–95. http://dx.doi.org/10.1016/S2468-1253(16)30024-3
- Abdul Basit S, Dawood A, Ryan J, Gish R. Tenofovir alafenamide for the treatment of chronic hepatitis B virus infection. Expert Rev Clin Pharmacol. 2017;10(7):707–16. http://dx.doi.org/10.1080/17512433.2017.1323633
- World Health Organization. 3: Antiretroviral agents – Therapeutic use. In: World Health Organization, editor. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Chapter 4: Clinical Guidelines Antiretroviral Therapy, 2nd ed. Geneva: World Health Organization; 2016. p. 72–190.
- WHO. Transition to new antiretroviral drugs in HIV programmes: Clinical and programatic considerations. Geneva: WHO; 2017.
- De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharmacol. 2016;119(1):1–7. http://dx.doi.org/10.1016/j.bcp.2016.04.015