REVIEW ARTICLE
Imaging of Renal Angiomyolipomatosis
Federico Greco, Carlo Augusto Mallio, Vincenzo Cirimele, Pasquale D’Alessio, Bruno Beomonte Zobel, Rosario Francesco Grasso
Unit of Diagnostic Imaging, Università Campus Bio-Medico di Roma, Rome, Italy
Abstract
Angiomyolipoma is a type of benign renal tumor. It is sporadic and isolated in 80% of cases. The remaining 20% is associated with tuberous sclerosis complex or pulmonary lymphangioleiomyomatosis. Generally, angiomyolipomas manifest themselves as angiomyolipomatosis, in which the angiomyolipomas are larger, bilateral, and widespread. Understanding whether angiomyolipomas are present in the context of angiomyolipomatosis is of considerable importance because it might be associated with malignant lesions. This article provides an overview of the radiological features of renal angiomyolipomatosis under different imaging techniques such as ultrasound, computed tomography, and magnetic resonance.
Keywords: angiomyolipoma; lymphangioleiomyomatosis; PEComa; renal angiomyolipomatosis; tuberous sclerosis
Received: 05 September 2018;
Accepted after revision: 21 October 2018;
Published: 14 November 2018
Author for correspondence: Federico Greco, Unit of Diagnostic Imaging, Università Campus Bio-Medico di Roma, Via Alvaro del Portillo, 21, 00128, Rome, Italy. Email:
federico.greco@unicampus.itHow to cite: Greco F, Mallio CA, Cirimele V, D’Alessio P, Beomonte Zobel B, Grasso RF. Imaging of renal angiomyolipomatosis. J Ren Hepat Disord. 2018;2(2):10–19.
Doi: http://dx.doi.org/10.15586/jrenhep.2018.37Copyright: Greco F et al.
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
Angiomyolipoma (AML) is a type of benign renal tumor, with an estimated prevalence of 0.3–3% of all renal tumors and a greater female predilection (1, 2). It is characteristically a solid “triphasic” tumor composed of dysmorphic blood vessels, smooth muscle components, and mature adipose tissue which may be present in varying amounts (3). AML was once considered a hamartoma and, most recently, a choristoma; it is now considered a part of perivascular epithelioid cell tumors (PEComa) (4–6). PEComa are mesenchymal neoplasms formed by nests and sheets of epithelioid and spindle cells that show immunoreactivity for both smooth muscle and melanocytic markers (7). The PEComas now include AML, pulmonary clear cell “sugar” tumor and lymphangioleiomyomatosis (LAM), primary extrapulmonary sugar tumor, clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres, abdominopelvic sarcoma of perivascular epithelioid cells, and other neoplasms with similar characteristics (8). Renal angiomyolipomatosis is a common manifestation in patients with tuberous sclerosis (TS) and LAM, where AMLs are larger, multiple, almost always bilateral, and have a greater predisposition to bleeding. AML is sporadic and isolated in 80% of cases, while the remaining 20% is associated with tuberous sclerosis complex (TSC) or pulmonary LAM (9, 10). Radiologically, the sporadic AML is predominantly classified into classic (common) and fat-poor AML (uncommon). Fat-poor AML is further classified into three subtypes: hyperattenuating AML (approximately 4.5% of all AMLs), isoattenuating AML (rare), and AML with epithelial cyst (rare). Another type of sporadic AML is epithelioid AML (rare). Syndromic AML is subdivided into AML in TSC and AML in LAM (11).The majority (>80%) of AMLs are detected incidentally during imaging. Most patients are asymptomatic when AML is diagnosed (10). The most common presentation is spontaneous retroperitoneal hemorrhage, although this happens in less than 15% of cases (10). Other clinical presentations are anemia, hematuria, palpable mass, flank pain, urinary tract infection, or renal failure (12, 13). As most classic AMLs do not increase in size and remain asymptomatic, the management is conservative. However, some grow gradually, showing a growth rate of 5% or 0.19 cm per year (14, 15). Oesterling et al. (16) proposed an algorithm for the management of AML based on tumor size and symptoms. For small AML (≤ 4 cm), follow-up with ultrasound (US) imaging is recommended every 12 months; for small AML in symptomatic patients, arterial embolization or partial nephrectomy can be chosen although observation is often favored in clinical practice. Treatment is recommended for symptomatic patients with large tumors, especially if the AML has bled. In asymptomatic patients with large AML, follow-up with computed tomography (CT) or US is recommended (16). Other options introduced for AML treatment are transarterial ethanol and percutaneous ablation using cryoablation or radiofrequency (17–19).
In this article, we describe the radiological features of renal angiomyolipomatosis. A PubMed search was performed by a radiologist for the term “angiomyolipomatosis.” The research showed 20 articles published in a period from 1969 to 2013. A total of 10 articles were excluded: four in German, three in French, one in Russian, and two did not describe the radiological features of renal angiomyolipomatosis. The remaining 10 articles in English, Italian, and Spanish languages describing radiological features of renal angiomyolipomatosis were selected.
Angiomyolipomatosis in Tuberous Sclerosis Complex
Tuberous sclerosis is largely the result of loss-of-function mutations of TSC1 (9q34) or TSC2 (16p13.3) genes. In addition to conditions such as mental retardation and seizures, TSC is associated with AMLs, LAM, pulmonary multifocal micronodular hyperplasia, subependymal giant cell tumors, cutaneous angiofibromas, and cardiac rabdomyomas (20). AMLs occur in 55–75% of patients with TS; AMLs in TS typically develop at a young age and are frequently multiple, almost always bilateral and larger in size, presenting as angiomyolipomatosis (Figure 1) (11, 21). Patients with TSC are more likely to show multiple, bilateral, and larger AMLs than AMLs in sporadic cases (22, 23). Most of the AMLs in TSC manifest as the classic type, while fat-poor AMLs are found in over one-third of these patients. Fat-poor AMLs in TSC tend to be larger than those of the sporadic form (24).
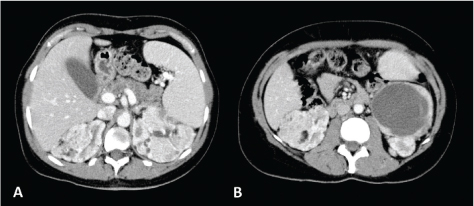
Figure 1. CT axial scan of the abdomen during venous phase of a 45-year-old woman with TS showing the presence of renal angiomyolipomatosis (A and B) and caliectasia at the level of the left upper calyceal group (A). Furthermore, a cystic lesion with solid peripheral tissue indissociable from the left inferior renal pole is evident (B). At the follow-up CT scan performed approximately 6 months later, the cystic lesion showed an increase of the solid component. Consequently, the patient underwent left nephrectomy and tumorectomy. Histological examination revealed the diagnosis of dedifferentiated liposarcoma.
As renal cell carcinoma may occur in patients with TSC, renal masses without visible characteristic adipose tissue may require a percutaneous biopsy or closer follow-up (15). Patients with TSC have also shown the presence of epithelioid AML and AML with epithelial cysts; TSC patients are more likely to show these two variants of AML compared to AMLs found sporadically (25, 26). Epithelioid AML shows variable biological behavior including malignancy; in fact, during adulthood, it can infiltrate adjacent tissue or metastasize to the lungs, liver, peritoneum, or bone (27, 28).
As patients with TSC risk premature loss of nephrons due to increasing numbers and dimensions of cysts and AMLs, selective arterial embolization, percutaneous ablation, or partial nephrectomy are preferred conservative therapies for the treatment of these lesions (29). Moreover, these patients present a high risk of spontaneous hemorrhage; AML >4 cm and AML aneurysms >0.5 cm are risk factors for AML hemorrhage (30, 31). Approximately 43% of patients with TSC may have recurrent AML bleeding, which is not usually seen in sporadic AMLs (32, 33). Indeed, angiomyolipomatosis is often associated with multiple spontaneous bleeding events. The mTOR inhibitor sirolimus allows the prevention of tumor growth and recurrence of bleeding in patients with TSC by inhibiting the activation of the mTOR pathway (34). Transcatheter embolization is an effective treatment for controlling bleeding in the acute context and can be performed in combination with surgery (17).
Angiomyolipomatosis in Lymphangioleiomyomatosis
Renal angiomyolipomatosis can be detected in patients with LAM, a rare disease characterized by destructive cystic changes in the lungs. Sporadic LAM manifests itself in one in 400,000 adult females; it may also happen in TSC, occurring in 30–40% of adult females and rarely in males and children (11, 35). In addition to renal AMLs, LAM presents other disorders including lymphangioleiomyomas, abdominal lymphadenopathy, and chylous ascites, and an increase in the frequency of meningioma (11, 35–38). As well as sporadic AMLs, the guidelines for LAM patients with AMLs recommend US examination per year for small AMLs (<4 cm), while larger AMLs and AMLs with aneurysms of 5 mm or greater diameter should be checked twice a year with US examination. The treatments of choice for a bleeding AML are renal arterial embolization and partial nephrectomy. Furthermore, the mTOR inhibitor sirolimus reduces the volume of AML (34, 35, 39).
Imaging Features of Angiomyolipomatosis
Several studies have described the radiological features of angiomyolipomatosis; for this review, we analyzed the radiological features described in several clinical cases. Imaging features of the cases of renal angiomyolipomatosis described in the literature are listed in Table 1 (40–49).
Table 1. Imaging features of cases of renal angiomyolipomatosis
| References |
Imaging method |
Imaging features |
| Segal et al. (40) |
Angiography |
Hypervascular
Renal enlargement
Deformed calyces |
| Ahuja et al. (41) |
Radiography |
Thick mass of soft tissue with many thin cloud-like calcification figures |
| Excretory urogram |
Marked congestion and stenosis of the left ureteral outlet
Left kidney well delimited
Right calico-pielic cavities deformed
Right kidney not well defined |
| Angiography |
Prominent vascular tortuosity with aneurysmal dilatation at lower pole of the left kidney
Altered vascular architecture with peripheral ectasies and microaneurysm of the right kidney; furthermore, the lower pole showed a region with a roundish morphology, highly vascularized, surrounded by wide arteries and a number of blood pools
Delayed passage time |
| Kalra et al. (42) |
Ultrasound |
Dimensional increase of the kidneys
Multiple bilateral echogenic masses |
| Contrast enhancement computed tomography |
Numerous irregular hypodense areas of adipose tissue density
Numerous isodense to hyperdense areas varying from soft tissue density to blood density
Poor excretion of contrast medium |
| Granata et al. (43) |
Ultrasound and color-power-doppler (first patient) |
Dimensional increase of the kidneys
Hyperechogenic parenchyma
No evidence of cortico-medullary differentiation
Absence of localized hypervascularization areas
Four renal cysts
Hypoechoic area in the right upper pole |
| Contrast enhancement magnetic resonance imaging (first patient) |
Coarse localized mass at the right upper pole of difficult interpretation. The differential diagnosis was with fat-poor AML, epithelioid AML, and renal cell carcinoma;
biopsy examination showed a diagnosis of fat-poor AML |
| Ultrasound (second patient) |
Dimensional increase of the kidneys
Irregular profiles of the kidneys
Structural subversion of the renal parenchyma with numerous and coarse nodules that alter to cysts |
| Magnetic resonance imaging (second patient) |
Numerous bilateral AMLs |
| Liu et al. (44) |
Unenhanced computed tomography |
Huge bilateral masses consisting primarily of adipose tissue |
| Ponce Díaz-Reixa et al. (45) |
Ultrasound and contrast enhancement computed tomography (first patient) |
Bilateral AMLs |
| Ultrasound (second patient) |
Nodule of the right kidney isoecogenous compared to perirenal adipose tissue |
| Contrast enhancement computed tomography (second patient) |
Mass at the right kidney, with heterogeneous appearance and adipose areas in the context, highly suggestive of AML
Histological and immunohistochemical examination confirmed the diagnosis of AML |
| Computed tomography (third patient) |
Mass at the right kidney, compatible with AML
Histological examination of the tumor demonstrated AML with epithelioid areas with infiltration of two lymph nodes that showed the same histological diagnosis |
| Er et al. (46) |
Unenhanced computed tomography |
Bilateral, renal masses (massive in the right kidney), with the density of adipose tissue |
| Incedayi et al. (47) |
Ultrasound |
Multiple bilateral hyperechogenic renal masses |
| Unenhanced computed tomography |
Massive renal masses
Fluid accumulation and high-density areas at the right kidney, developed following the previous hemorrhage |
| Stallone et al. (48) |
Ultrasound |
Multiple renal hyperechogenic lesions |
| Contrast enhancement computed tomography |
Multiple renal lesions, describing a framework compatible with angiomyolipomatosis. Histological analysis confirmed the diagnosis of angiomyolipomatosis |
| Vergnani et al. (49) |
Unenhanced computed tomography |
Widespread angiomyolipomatosis in the context of which fat components and soft-tissue tumor components were evident; hematomas were also present |
| AML, angiomyolipoma. |
Renal angiomyolipomatosis generally occurs with multiple and diffuse AMLs, bilaterally localized. Often the masses extend almost entirely covering the abdomen, displacing the intestine. Signs of previous bleeding may be present. Renal cysts could also be detected. In AMLs with a predominantly fatty component, the adipose tissue localized within these lesions assumes fundamental importance in the diagnosis. On US examination, it appears hyperechoic compared to renal cortex. In CT examination, it appears as hypodense area of adipose tissue density, mixed, or with soft-tissue attenuation due to vascular or smooth muscle components, hemorrhage or fibrosis (2).
Bosniak described the angiographic features of AML. The author observed three patterns in particular: aneurysmal and tortuous vessels, berry-like aneurysms, and slow-flowing vessels with contrast medium retention (50).
On magnetic resonance imaging (MRI), it appears isointense compared with fat on T1-weighted images; moreover, with the use of in-phase and opposed-phase imaging, AMLs with predominant adipose component show the characteristic India ink artifact that appears at the interface between the lesion and the normal renal parenchyma on opposed-phase T1-weighted images. In T2-weighted images, however, the intensity can be variable, depending on the amount of adipose tissue present in the lesion, resulting homogeneously high in AMLs with a higher adipose component (Figure 2) (51–54).
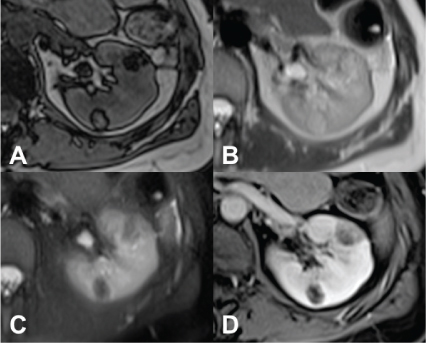
Figure 2. MRI axial scan of the abdomen shows two AMLs of the left kidney. (A) Opposed-phase shows the characteristic India ink artifact of the AMLs. (B) AMLs appear hyperintense on T2-weighted images and (C) hypointense on T2-weighted images with fat suppression. (D) T1-weighted image with fat suppression shows contrast enhancement of the AMLs.
The diagnosis is more difficult if there is the presence of fat-poor AML, epithelial AML, and AML with epithelial cysts, as we must discriminate these lesions from malignant lesions, such as renal cell carcinoma or the same epithelioid AML with malignant biological behavior, being able to be present in renal angiomyolipomatosis. For example, it is difficult to differentiate fat-poor AML from other solid tumors, especially renal cell carcinoma. In this case, double-echo gradient-echo chemical-shift MRI could be used in which the values of the signal intensity are measured on the renal lesion and on the spleen in on-phase and opposed-phase T1-weighted gradient-echo MRI (54). The presence of small calcifications within the lesion, which can be easily detected with CT, is considered to be suggestive of renal cell carcinoma (55). Furthermore, central necrosis is indicative of renal cell carcinoma, this being frequently present in medium-to-large clear cell renal cell carcinoma and very rare in AML. In fat-poor AML, the low amounts of adipose tissue can be detected on opposed-phase and in-phase imaging. It also appears homogeneously hypointense on T2-weighted images (53).
Finally, even contrast enhancement US can be used in the differential diagnosis between malignant and benign renal lesions (56). In a retrospective study, Lu et al. found a slow centripetal enhancement in the cortical phase and a homogeneous enhancement in the peak phase in fat-poor renal AML (57).
Radiologic Diagnosis of Renal Angiomyolipoma
Jinzaki et al. proposed an AML classification in which clinical features, radiologic features, and pathologic features coexist. This section focuses on the radiologic characteristics indicated in the AML classification of Jinzaki et al. (11).
Classic angiomyolipoma
Classic AML is a subtype of triphasic AML. The typical characteristic of classic AML is the presence of abundant adipose tissue (11). This AML almost always appears markedly hyperechoic compared to the renal parenchyma. In addition, 21–33% of AMLs smaller than 3 cm show acoustic shadowing (58, 59). The fat present in AML can be identified on unenhanced CT with a region of interest (ROI) showing an attenuation less than −10 HU (Figure 3) (50, 60, 61). The CT features of classic AML vary due to variable amounts of the three components present in the lesion (11). Furthermore, intralesional hemorrhage may be present, especially in tumors larger than 4 cm (62). MRI can be used to diagnose AML also by detecting fat cells; India ink artifact visible with a loss of signal at the boundary between the mass and the renal parenchyma is indicative of AML (52).
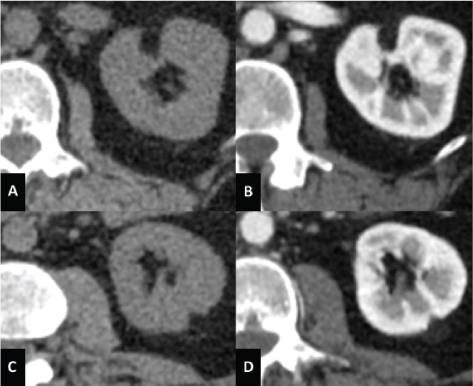
Figure 3. Unenhanced CT axial scan of the abdomen (A and C) and CT of the abdomen during arterious phase (B and D) of a 53-year-old woman showing the presence of classic AMLs, recognizable by the adipose component of the lesion.
Differential diagnosis of classic AML is with renal cell carcinoma, Wilms tumor, and retroperitoneal liposarcoma and teratoma (11).
Fat-poor angiomyolipoma
Fat-poor AMLs are those triphasic AMLs that contain too little fat to be identified with unenhanced CT (4, 63). There are three subtypes of fat-poor AML; their subdivision is based on the number of fat cells and their distribution within the lesion; they are hyperattenuating and isoattenuating AMLs, and AML with epithelial cysts (64).
Hyperattenuating angiomyolipoma
Hyperattenuating AML makes up about 4–5% of all AMLs (65). This subtype of fat-poor AML is generally small, with an average of 3 cm of diameter, and accounts for only 4% (3–10% range) of fat cells (65–67). As there is an abundant amount of smooth muscle component, they present characteristics similar to those of smooth muscle: they appear hyperattenuating compared to renal parenchyma on unenhanced CT (usually greater than 45 HU); T1-hypointense and T2-hypointense on MRI; no signal loss on fat-suppressed pulse sequences, and chemical shift suppression; and isoechoic on US, with one study suggesting could be hyperechoic (65–67). Differential diagnosis of hyperattenuating AML is with renal cell carcinoma (typically the papillary renal cell carcinoma), metastases, oncocytoma, lymphoma, metanephric adenoma, and leiomyoma (63, 68).
Isoattenuating angiomyolipoma
Isoattenuating AMLs possess CT attenuations similar to those of the renal parenchyma on unenhanced CT. This type of AML does not possess regions of adipose tissue attenuation at unenhanced CT. In particular, fat cells are dispersed between smooth muscle and vessel components, too few to be detected with imaging but in sufficient quantities to reduce the overall attenuation compared to hyperattenuating AML (69). On MRI, this subtype of fat-poor AML appears typically T2-hypointense. This feature is given by its smooth muscle component (70). Furthermore, Jinzaki et al. claim that isoattenuating AML characteristics on all MRI pulse sequences are not well known because it is a rare lesion; this lesion may or may not show signal loss on fat-suppressed pulse sequences; the loss of signal depends both on the quantity and the distribution of fat cells within the lesion (11). It also shows chemical shift suppression (54, 70). Jinzaki et al. also state that, based on their experience, isoattenuating AML appears slightly hyperechoic on US (11). Differential diagnosis of isoattenuating AML is with renal cell carcinoma (11).
Angiomyolipoma with epithelial cysts
AML with epithelial cysts is a very rare variant of the fat-poor AML which contains epithelial-lined cysts. These AMLs have very few or no fat cells (71). This subtype of AML is benign and more common in female (69, 71–74). AML with epithelial cysts contains smooth muscle component, which represents the predominant component, and epithelial cysts and subepithelial stroma, which are typical of this subtype of fat-poor AML (69, 72). The imaging features of AML with epithelial cyst are not fully understood. A case was described in which the lesion presented a small cyst, and a non-cystic part that enhanced homogeneously. This lesion appeared hyperattenuating on unenhanced CT and, on MRI, T2-hypointense for the smooth muscle component (71). Another case described a multilocular cystic mass with the cystic component separated from that smooth muscle (73). Differential diagnosis is with multilocular cystic renal cell carcinoma, multilocular cyst, cystic nephroma, and a mixed epithelial and stromal tumor (73, 75).
Epithelioid angiomyolipoma
Epithelioid AML is a subtype of extremely rare potentially malignant AML (3, 25). Male and female are equally affected and the average age is 38 years (3). Approximately one-third has local extension or metastasis at diagnosis (76). Epithelioid AML contains numerous atypical epithelioid muscle cells; in most of these lesions there are few or no fat cells (25, 77, 78). This AML subtype typically appears as large masses (≥5 cm in size) with intralesional hemorrhage and necrosis; it can also be detected as spontaneous perirenal hematoma (79–85). These lesions may show small foci of adipose tissue on CT or MRI; moreover, epithelioid AML appears hyperattenuating on unenhanced CT (typically greater than 45 HU) and T2-hypointense (due to epithelioid muscle component) (83, 84). Furthermore, this AML subtype may appear as solid masses that enhance homogeneously or heterogeneously or as multilocular cystic masses (84). Differential diagnosis of epithelioid AML is with renal cell carcinoma and cystic renal cell carcinoma (25, 77, 84).
New Radiologic Classification of Renal Angiomyolipoma
Song et al. classified renal AML into fat-rich, fat-poor, and fat-invisible AML based on the amount of fat detected by CT or MRI (86). Fat-rich AML was identified by attenuation value less than or equal to −10 HU obtained by placing a ROI in the most hypodense area of the lesion (61). When the most hypodense area showed attenuation value greater than −10 HU, the chemical shift imaging was evaluated. Tumor-to-spleen ratio and signal intensity index were calculated using the values obtained by placing the ROI in the most hypointense area on opposed-phase images. Fat-poor AML was detected when the tumor-to-spleen ratio was <0.71 or when the signal intensity index value was >16.5%. Fat-invisible AML was detected when the tumor-to-spleen ratio was ≥0.71 and when the signal intensity index value was ≤16.5% (86). Both fat-poor AML and fat-invisible AML exhibit attenuation values greater than –10 HU on unenhanced CT. Song et al. showed that the attenuation value of fat-invisible AML detected in the ROI located in the most hypodense area of the lesion was greater than that of the fat-poor AML (86).
Conclusion
Renal angiomyolipomatosis is a common manifestation in patients with TS and LAM. AMLs are larger, multiple, almost always bilateral, and have a greater predisposition to bleeding, which is the reason why follow-up must be performed in these patients. Furthermore, specific subtypes of AML, such as poor-fat AML, are difficult to distinguish from malignant lesions. For this reason, further imaging examinations must be performed to obtain further information on the nature of the lesions.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int. 2004 Sep;66(3):924–34. https://doi.org/10.1111/j.1523-1755.2004.00838.x
- Wagner BJ, Wong-You-Cheong JJ, Davis CJ, Jr. Adult renal hamartomas. Radiographics. 1997 Jan–Feb;17(1):155–69. https://doi.org/10.1148/radiographics.17.1.9017806
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization classification of tumors: Pathology and genetics. Tumors of the urinary system and male genital organs. Lyon: IARC Press; 2004.
- Lane BR, Aydin H, Danforth TL, Zhou M, Remer EM, Novick AC, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: Classic, fat poor, tuberous sclerosis associated and epithelioid. J Urol. 2008 Sep;180(3):836–43. https://doi.org/10.1016/j.juro.2008.05.041
- Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: Benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000 Jan;7(1):47–66. https://doi.org/10.1097/00125480-200007010-00007
- Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, et al. Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996 Jun;20(6):722–30. https://doi.org/10.1097/00000478-199606000-00010
- Hornick JL, Fletcher CD. PEComa: What do we know so far? Histopathology. 2006 Jan;48(1):75–82. https://doi.org/10.1111/j.1365-2559.2005.02316.x
- Thway K, Fisher C. PEComa: Morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015 Oct;19(5):359–68. https://doi.org/10.1016/j.anndiagpath.2015.06.003
- Fittschen A, Wendlik I, Oeztuerk S, Kratzer W, Akinli AS, Haenle MM, et al. Prevalence of sporadic renal angiomyolipoma: A retrospective analysis of 61,389 in- and out-patients. Abdom Imaging. 2014 Oct;39(5):1009–13. https://doi.org/10.1007/s00261-014-0129-6
- Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. Update on the diagnosis and management of renal angiomyolipoma. J Urol. 2016 Apr;195(4 Pt 1):834–46. https://doi.org/10.1016/j.juro.2015.07.126
- Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: A radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014 Jun;39(3):588–604. https://doi.org/10.1007/s00261-014-0083-3
- Halpenny D, Snow A, McNeill G, Torreggiani WC. The radiological diagnosis and treatment of renal angiomyolipoma-current status. Clin Radiol. 2010 Feb;65(2):99–108. https://doi.org/10.1016/j.crad.2009.09.014
- Logue LG, Acker RE, Sienko AE. Best cases from the AFIP: Angiomyolipomas in tuberous sclerosis. Radiographics. 2003 Jan–Feb;23(1):241–6. https://doi.org/10.1148/rg.231025109
- Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: Comparison of sporadic and tuberous sclerosis complex-associated forms. Urology. 2008 Nov;72(5):1077–82. https://doi.org/10.1016/j.urology.2008.07.049
- Lemaitre L, Robert Y, Dubrulle F, Claudon M, Duhamel A, Danjou P, et al. Renal angiomyolipoma: Growth followed up with CT and/or US. Radiology. 1995 Dec;197(3):598–602. https://doi.org/10.1148/radiology.197.3.7480725
- Oesterling JE, Fishman EK, Goldman SM, Marshall FF. The management of renal angiomyolipoma. J Urol. 1986 Jun;135(6):1121–4. https://doi.org/10.1016/S0022-5347(17)46013-7
- Takebayashi S, Horikawa A, Arai M, Iso S, Noguchi K. Transarterial ethanol ablation for sporadic and non-hemorrhaging angiomyolipoma in the kidney. Eur J Radiol. 2009 Oct;72(1):139–45. https://doi.org/10.1016/j.ejrad.2008.06.017
- Castle SM, Gorbatiy V, Ekwenna O, Young E, Leveillee RJ. Radiofrequency ablation (RFA) therapy for renal angiomyolipoma (AML): An alternative to angio-embolization and nephron-sparing surgery. BJU Int. 2012 Feb;109(3):384–7. https://doi.org/10.1111/j.1464-410X.2011.10376.x
- Byrd GF, Lawatsch EJ, Mesrobian HG, Begun F, Langenstroer P. Laparoscopic cryoablation of renal angiomyolipoma. J Urol. 2006 Oct;176(4 Pt 1):1512–16; discussion 6. https://doi.org/10.1016/j.juro.2006.06.013
- Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: The past, the present and the future. Virchows Arch. 2008 Feb;452(2):119–32. https://doi.org/10.1007/s00428-007-0509-1
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006 Sep 28;355(13):1345–56. https://doi.org/10.1056/NEJMra055323
- Castagnetti M, Vezzu B, Laverda A, Zampieri S, Rigamonti W. Urological counseling and followup in pediatric tuberous sclerosis complex. J Urol. 2007 Nov;178(5):2155–9. https://doi.org/10.1016/j.juro.2007.07.058
- Ewalt DH, Sheffield E, Sparagana SP, Delgado MR, Roach ES. Renal lesion growth in children with tuberous sclerosis complex. J Urol. 1998 Jul;160(1):141–5. https://doi.org/10.1016/S0022-5347(01)63072-6
- Lemaitre L, Claudon M, Dubrulle F, Mazeman E. Imaging of angiomyolipomas. Semin Ultrasound CT MR. 1997 Apr;18(2):100–14. https://doi.org/10.1016/S0887-2171(97)90054-8
- Eble JN, Amin MB, Young RH. Epithelioid angiomyolipoma of the kidney: A report of five cases with a prominent and diagnostically confusing epithelioid smooth muscle component. Am J Surg Pathol. 1997 Oct;21(10):1123–30. https://doi.org/10.1097/00000478-199710000-00001
- Aydin H, Magi-Galluzzi C, Lane BR, Sercia L, Lopez JI, Rini BI, et al. Renal angiomyolipoma: Clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol. 2009 Feb;33(2):289–97. https://doi.org/10.1097/PAS.0b013e31817ed7a6
- Schieda N, Kielar AZ, Al Dandan O, McInnes MD, Flood TA. Ten uncommon and unusual variants of renal angiomyolipoma (AML): Radiologic-pathologic correlation. Clin Radiol. 2015 Feb;70(2):206–20. https://doi.org/10.1016/j.crad.2014.10.001
- Tirumani SH, Shinagare AB, Hargreaves J, Jagannathan JP, Hornick JL, Wagner AJ, et al. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am J Roentgenol. 2014 Feb;202(2):252–8. https://doi.org/10.2214/AJR.13.10909
- Geller E, Kochan PS. Renal neoplasms of childhood. Radiol Clin North Am. 2011 Jul;49(4):689–709, vi. https://doi.org/10.1016/j.rcl.2011.05.003
- Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: Relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002 Oct;225(1):78–82. https://doi.org/10.1148/radiol.2251011477
- Pirson Y. Tuberous sclerosis complex-associated kidney angiomyolipoma: From contemplation to action. Nephrol Dial Transplant. 2013 Jul;28(7):1680–5. https://doi.org/10.1093/ndt/gft009
- Kothary N, Soulen MC, Clark TW, Wein AJ, Shlansky-Goldberg RD, Crino PB, et al. Renal angiomyolipoma: Long-term results after arterial embolization. J Vasc Interv Radiol. 2005 Jan;16(1):45–50. https://doi.org/10.1097/01.RVI.0000143769.79774.70
- Lee W, Kim TS, Chung JW, Han JK, Kim SH, Park JH. Renal angiomyolipoma: Embolotherapy with a mixture of alcohol and iodized oil. J Vasc Interv Radiol. 1998 Mar-Apr;9(2):255–61. https://doi.org/10.1016/S1051-0443(98)70266-0
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008 Jan 10;358(2):140–51. https://doi.org/10.1056/NEJMoa063564
- Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al. European respiratory society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010 Jan;35(1):14–26. https://doi.org/10.1183/09031936.00076209
- Avila NA, Kelly JA, Chu SC, Dwyer AJ, Moss J. Lymphangioleiomyomatosis: Abdominopelvic CT and US findings. Radiology. 2000 Jul;216(1):147–53. https://doi.org/10.1148/radiology.216.1.r00jl42147
- Maziak DE, Kesten S, Rappaport DC, Maurer J. Extrathoracic angiomyolipomas in lymphangioleiomyomatosis. Eur Respir J. 1996 Mar;9(3):402–5. https://doi.org/10.1183/09031936.96.09030402
- Kenerson H, Folpe AL, Takayama TK, Yeung RS. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol. 2007 Sep;38(9):1361–71. https://doi.org/10.1016/j.humpath.2007.01.028
- Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008 Jan 10;358(2):200–3. https://doi.org/10.1056/NEJMc072500
- Segal AJ, Spataro RF, Barbaric ZL. Adult polycystic kidney disease: A review of 100 cases. J Urol. 1977 Nov;118(5):711–13. https://doi.org/10.1016/S0022-5347(17)58169-0
- Ahuja S, Loffler W, Wegener OH, Ernst H. Tuberous sclerosis with angiomyolipoma and metastasized hypernephroma. Urology. 1986 Nov;28(5):413–19. https://doi.org/10.1016/0090-4295(86)90076-2
- Kalra OP, Verma PP, Kochhar S, Jha V, Sakhuja V. Bilateral renal angiomyolipomatosis in tuberous sclerosis presenting with chronic renal failure: Case report and review of the literature. Nephron. 1994 68(2):256–8. https://doi.org/10.1159/000188267
- Granata A, Sessa A, Pitangolo F, Spata C, Sicurezza E, Costantino G, et al. [Chronic renal failure and tuberous sclerosis. Report of two clinical cases]. Minerva Urol Nefrol. 2002 Dec;54(4):243–8.
- Liu H, Cooke K, Frager D. Bilateral massive renal angiomyolipomatosis in tuberous sclerosis. AJR Am J Roentgenol. 2005 Oct;185(4):1085–6. https://doi.org/10.2214/AJR.04.1906
- Ponce Diaz-Reixa J, Barbagelata Lopez A, Romero Selas E, Marcos Rodriguez P, Sanchez Rodriguez-Losada J, Alvarez Castelo L, et al. [Renal angiomyolipomatosis and pulmonary lymphangiomyomatosis. Its relationship with Bourneville syndrome]. Actas Urol Esp. 2006 Apr;30(4):386–93. https://doi.org/10.1016/S0210-4806(06)73462-3
- Er A, Yildirim CA. Giant renal angiomyolipomatosis in association with pulmonary lymphangiomyomatosis. Intern Med J. 2010 May;40(5):384–5. https://doi.org/10.1111/j.1445-5994.2010.02218.x
- Incedayi M, Sonmez G, Basekim C. Massive bilateral renal angiomyolipomatosis and multifocal micronodular pneumocytes hyperplasia associated with tuberous sclerosis: A case report. Wien Klin Wochenschr. 2011 Nov;123(21–22):674–6. https://doi.org/10.1007/s00508-011-0073-1
- Stallone G, Infante B, Tartaglia L, Bruno F, Gesualdo L, Grandaliano G. Renal angiomyolipomatosis and Kaposi’s sarcoma: A possible link disrupted by sirolimus. Intern Emerg Med. 2012 Sep;7 (Suppl 2):S127–9. https://doi.org/10.1007/s11739-012-0833-6
- Vergnani J, Graham JD. Renal angiomyolipomatosis in a patient with tuberous sclerosis complex. J Am Osteopath Assoc. 2013 Jan;113(1):104.
- Bosniak MA. Angiomyolipoma (hamartoma) of the kidney: A preoperative diagnosis is possible in virtually every case. Urol Radiol. 1981;3(3):135–42. https://doi.org/10.1007/BF02938781
- Rofsky NM, Bosniak MA. MR imaging in the evaluation of small (< or =3.0 cm) renal masses. Magn Reson Imaging Clin N Am. 1997 Feb;5(1):67–81.
- Israel GM, Hindman N, Hecht E, Krinsky G. The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR Am J Roentgenol. 2005 Jun;184(6):1868–72. https://doi.org/10.2214/ajr.184.6.01841868
- Pedrosa I, Sun MR, Spencer M, Genega EM, Olumi AF, Dewolf WC, et al. MR imaging of renal masses: Correlation with findings at surgery and pathologic analysis. Radiographics. 2008 Jul–Aug;28(4):985–1003. https://doi.org/10.1148/rg.284065018
- Kim JK, Kim SH, Jang YJ, Ahn H, Kim CS, Park H, et al. Renal angiomyolipoma with minimal fat: Differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology. 2006 Apr;239(1):174–80. https://doi.org/10.1148/radiol.2391050102
- Dillon RC, Friedman AC, Miller FH. MR signal intensity calculations are not reliable for differentiating renal cell carcinoma from lipid poor angiomyolipoma. Radiology. 2010 Oct;257(1):299–300. https://doi.org/10.1148/radiol.100520
- Blomley M, Claudon M, Cosgrove D. WFUMB safety symposium on ultrasound contrast agents: Clinical applications and safety concerns. Ultrasound Med Biol. 2007 Feb;33(2):180–6. https://doi.org/10.1016/j.ultrasmedbio.2006.07.007
- Lu Q, Wang W, Huang B, Li C, Li C. Minimal fat renal angiomyolipoma: The initial study with contrast-enhanced ultrasonography. Ultrasound Med Biol. 2012 Nov;38(11):1896–901. https://doi.org/10.1016/j.ultrasmedbio.2012.07.014
- Siegel CL, Middleton WD, Teefey SA, McClennan BL. Angiomyolipoma and renal cell carcinoma: US differentiation. Radiology. 1996 Mar;198(3):789–93. https://doi.org/10.1148/radiology.198.3.8628873
- Jinzaki M, Ohkuma K, Tanimoto A, Mukai M, Hiramatsu K, Murai M, et al. Small solid renal lesions: Usefulness of power Doppler US. Radiology. 1998 Nov;209(2):543–50. https://doi.org/10.1148/radiology.209.2.9807587
- Bosniak MA, Megibow AJ, Hulnick DH, Horii S, Raghavendra BN. CT diagnosis of renal angiomyolipoma: The importance of detecting small amounts of fat. AJR Am J Roentgenol. 1988 Sep;151(3):497–501. https://doi.org/10.2214/ajr.151.3.497
- Simpson E, Patel U. Diagnosis of angiomyolipoma using computed tomography-region of interest < or =-10 HU or 4 adjacent pixels < or =-10 HU are recommended as the diagnostic thresholds. Clin Radiol. 2006 May;61(5):410–16. https://doi.org/10.1016/j.crad.2005.12.013
- Corr P, Yang WT, Tan I. Spontaneous haemorrhage from renal angiomyolipomata. Australas Radiol. 1994 May;38(2):132–4. https://doi.org/10.1111/j.1440-1673.1994.tb00153.x
- Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008 Oct;249(1):16–31. https://doi.org/10.1148/radiol.2491070783
- Milner J, McNeil B, Alioto J, Proud K, Rubinas T, Picken M, et al. Fat poor renal angiomyolipoma: Patient, computerized tomography and histological findings. J Urol. 2006 Sep;176(3):905–9. https://doi.org/10.1016/j.juro.2006.04.016
- Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma K, Kurata T, Shinmoto H, et al. Angiomyolipoma: Imaging findings in lesions with minimal fat. Radiology. 1997 Nov;205(2):497–502. https://doi.org/10.1148/radiology.205.2.9356635
- Hafron J, Fogarty JD, Hoenig DM, Li M, Berkenblit R, Ghavamian R. Imaging characteristics of minimal fat renal angiomyolipoma with histologic correlations. Urology. 2005 Dec;66(6):1155–9. https://doi.org/10.1016/j.urology.2005.06.119
- Trigaux JP, Pauls C, Van Beers B. Atypical renal hamartomas: Ultrasonography, computed tomography, and angiographic findings. J Clin Ultrasound. 1993 Jan;21(1):41–4. https://doi.org/10.1002/jcu.1870210109
- Silverman SG, Mortele KJ, Tuncali K, Jinzaki M, Cibas ES. Hyperattenuating renal masses: Etiologies, pathogenesis, and imaging evaluation. Radiographics. 2007 Jul–Aug;27(4):1131–43. https://doi.org/10.1148/rg.274065147
- Fine SW, Reuter VE, Epstein JI, Argani P. Angiomyolipoma with epithelial cysts (AMLEC): A distinct cystic variant of angiomyolipoma. Am J Surg Pathol. 2006 May;30(5):593–9. https://doi.org/10.1097/01.pas.0000194298.19839.b4
- Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A. Small (<4 cm) renal mass: Differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology. 2012 Apr;263(1):160–8. https://doi.org/10.1148/radiol.12111205
- Rosenkrantz AB, Hecht EM, Taneja SS, Melamed J. Angiomyolipoma with epithelial cysts: Mimic of renal cell carcinoma. Clin Imaging. 2010 Jan–Feb;34(1):65–8. https://doi.org/10.1016/j.clinimag.2009.04.026
- Davis CJ, Barton JH, Sesterhenn IA. Cystic angiomyolipoma of the kidney: A clinicopathologic description of 11 cases. Mod Pathol. 2006 May;19(5):669–74. https://doi.org/10.1038/modpathol.3800572
- Mikami S, Oya M, Mukai M. Angiomyolipoma with epithelial cysts of the kidney in a man. Pathol Int. 2008 Oct;58(10):664–7. https://doi.org/10.1111/j.1440-1827.2008.02287.x
- Armah HB, Yin M, Rao UN, Parwani AV. Angiomyolipoma with epithelial cysts (AMLEC): A rare but distinct variant of angiomyolipoma. Diagn Pathol. 2007 Mar 21;2:11. https://doi.org/10.1186/1746-1596-2-11
- Greco F, Faiella E, Santucci D, De Lisi D, Lo Vullo G, Beomonte Zobel B, et al. Ultrasound imaging of cystic nephroma. J Kidney Cancer VHL. 2017;4(3):1–9. https://doi.org/10.15586/jkcvhl.2017.79.
- Tsai CC, Wu WJ, Li CC, Wang CJ, Wu CH, Wu CC. Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma: A clinicopathologic analysis of cases and literature review. Kaohsiung J Med Sci. 2009 Mar;25(3):133–40. https://doi.org/10.1016/S1607-551X(09)70052-X
- Park HK, Zhang S, Wong MK, Kim HL. Clinical presentation of epithelioid angiomyolipoma. Int J Urol. 2007 Jan;14(1):21–5. https://doi.org/10.1111/j.1442-2042.2006.01665.x
- Warakaulle DR, Phillips RR, Turner GD, Davies D, Protheroe AS. Malignant monotypic epithelioid angiomyolipoma of the kidney. Clin Radiol. 2004 Sep;59(9):849–52. https://doi.org/10.1016/j.crad.2004.02.009
- Bharwani N, Christmas TJ, Jameson C, Moat N, Sohaib SA. Epithelioid angiomyolipoma: Imaging appearances. Br J Radiol. 2009 Dec;82(984):e249–52. https://doi.org/10.1259/bjr/27259024
- Chen J, Wang P, Wang CJ, Cai SL, Ren GP, Li YY. Highly aggressive epithelioid renal angiomyolipoma with a very poor prognosis. Chin Med J (Engl). 2010 Mar 20;123(6):765–6.
- Radin R, Ma Y. Malignant epithelioid renal angiomyolipoma in a patient with tuberous sclerosis. J Comput Assist Tomogr. 2001 Nov–Dec;25(6):873–5. https://doi.org/10.1097/00004728-200111000-00008
- Hung MS, Chang JH, Chang CP, Tai HL. Massive epithelioid angiomyolipoma of the kidney in a young girl. Int J Urol. 2005 Nov;12(11):998–1000. https://doi.org/10.1111/j.1442-2042.2005.01194.x
- Froemming AT, Boland J, Cheville J, Takahashi N, Kawashima A. Renal epithelioid angiomyolipoma: Imaging characteristics in nine cases with radiologic-pathologic correlation and review of the literature. AJR Am J Roentgenol. 2013 Feb;200(2):W178–86. https://doi.org/10.2214/AJR.12.8776
- Tsukada J, Jinzaki M, Yao M, Nagashima Y, Mikami S, Yashiro H, et al. Epithelioid angiomyolipoma of the kidney: Radiological imaging. Int J Urol. 2013 Nov;20(11):1105–11. https://doi.org/10.1111/iju.12117
- Zhan R, Li YQ, Chen CY, Hu HY, Zhang C. Primary kidney malignant epithelioid angiomyolipoma: Two cases report and review of literature. Medicine (Baltimore). 2018 Aug;97(32):e11805. https://doi.org/10.1097/MD.0000000000011805
- Song S, Park BK, Park JJ. New radiologic classification of renal angiomyolipomas. Eur J Radiol. 2016 Oct;85(10):1835–42. https://doi.org/10.1016/j.ejrad.2016.08.012