REVIEW ARTICLE
The Role of Stearoyl-coenzyme A Desaturase 1 in Liver Development, Function, and Pathogenesis
Fatemeh Mohammadzadeh1, Vahid Hosseini2, Alireza Alihemmati3, Maghsod Shaaker1, Gholamali Mosayyebi4, Masoud Darabi1, Amir Mehdizadeh5
1Emergency Medicine Research Center Team, Department of Emergency Medicine, Tabriz University of Medical Sciences, Tabriz, Iran; 2Department of Biochemistry and Clinical Laboratories, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran; 3Department of Anatomical Sciences, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran; 4Liver and Gastrointestinal Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 5Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Stearoyl-coenzyme A desaturase 1 (SCD1) is a microsomal enzyme that controls fatty acid metabolism and is highly expressed in hepatocytes. SCD1 may play a key role in liver development and hepatic lipid homeostasis through promoting monounsaturated protein acylation and converting lipotoxic saturated fatty acids into monounsaturated fatty acids. Imbalanced activity of SCD1 has been implicated in fatty liver induction, inflammation and stress. In this review, the role of SCD1 in hepatic development, function and pathogenesis is discussed. Additionally, emerging novel therapeutic agents targeting SCD1 for the treatment of liver disorders are presented.
Keywords: hepatic lipogenesis; hydroxy pyridine; MK-8245; stearoyl-coenzyme A desaturase 1; SCD1
Received: 20 December 2018;
Accepted after Revision: 15 January 2019;
Published: 06 February 2019
Authors for correspondence: Masoud Darabi, Emergency Medicine Research Center Team, Department of Emergency Medicine, Tabriz University of Medical Sciences, Tabriz, Iran. Email:
darabim@tbzmed.ac.ir; Amir Mehdizadeh, Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. Email:
mehdizadeha@tbzmed.ac.irHow to cite: Mohammadzadeh F et al. The role of Stearoyl-coenzyme A Desaturase 1 in liver development, function and pathogenesis. J Ren Hepat Disord. 2019;3(1):15–22.
Doi: http://dx.doi.org/10.15586/jrenhep.2019.49Copyright: Mohammadzadeh F et al.
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
Stearoyl-coenzyme A desaturase 1 (SCD1) was discovered in 1988 when Ntambi and colleagues identified an mRNA transcript whose expression was highly induced during adipogenic differentiation (1). SCD1 is an iron-containing lipid-regulating enzyme that is highly expressed in the liver and is the main enzyme responsible for de novo synthesis of monounsaturated fatty acids (MUFAs). Palmitoyl-CoA and stearoyl-CoA are the main substrates of this enzyme, converting them into palmitoleoyl-CoA and oleoyl-CoA, respectively (2).
SCD1 is coded by its gene on the long arm of chromosome 24, in the sub-band 3 of region 24 (3). Promoter activity region is located within the initial 609 bp upstream of transcription initiation site which constitutes a CCAAT-box identified as a cis-element binding site. Sterol regulatory element-binding transcription factor 1 (SREBP-1c), liver X receptor (LXR), peroxisome proliferator-activated receptor alpha (PPAR-α) and CCAAT/enhancer-binding protein alpha (C/EBP-α) are among the most important transcription factors that bind to SCD1 promoter and control its gene expression (4). The pseudogene of SCD1, containing two premature stop codons downstream of the original start codon, is located on the short arm of chromosome 24 in the sub-band 32 of region 11 (5).
SCD1 protein is a microsomal enzyme containing four transmembrane domains in which both the N-terminus and C-terminus are located in the cytoplasm (Figure 1). Eight histidine residues on the single cytoplasmic loop and C-terminus are conserved and important for desaturase catalytic activity (6). Purified SCD1 protein migrates as a 37 kDa band by SDS gel electrophoresis (7, 8). As fatty acids are important components of phospholipids, triglycerides and esterified cholesterol, changes in SCD1 expression and activity can affect membrane stability, lipid metabolism and the amount of adipose tissue; consequent changes may be associated with obesity, fatty liver, cancers, diabetes and atherosclerosis (9). This review provides an overview of the role of SCD1 on various aspects of liver pathophysiology such as development, hepatic lipogenesis and inflammation. It also summarizes the role of novel small molecules targeting SCD1 as potential agents for the treatment of various liver disorders.
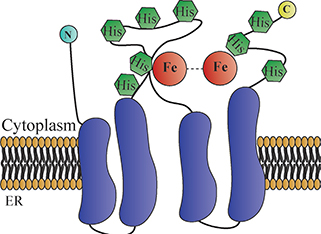
Figure 1. Stearoyl-coenzyme A desaturase 1 (SCD1) is an iron-containing transmembrane enzyme. SCD1 protein is exclusively localized on the ER membrane with both the N- and C-terminal domains stretched into the cytosol. It has four transmembrane helices (purple cylindrical shapes). The eight histidines (hexagonal shapes) on the single cytoplasmic loop and C-terminus are highly conserved, particularly regions surrounding the di-iron center. The cytosolic domain provides a structural frame for the regioselectivity and stereospecificity of the desaturation reaction (6).
SCD1 Activity Contributes to Liver Development through Protein Acylation
The Wnt family of proteins are signaling molecules that orchestrate numerous homeostatic events from embryonic development to adult tissue function (10). Their malfunction causes various hepatic abnormalities. The products of SCD1 can regulate Wnt trafficking and function through MUFA acylation or lipidation (Figure 2). Monounsaturated fatty acyl moieties render Wnts hydrophobic and insoluble in aqueous environment (11). During the embryonic stage, the inner layer, endoderm, is partitioned into three regions termed as foregut, midgut, and hindgut, with the foregut containing liver precursors. The liver and biliary tracts develop from the foregut at the 4th week of gestation (12). The intermediate germ layer, mesoderm, produces Wnt which contributes to the development of hindgut in the posterior endoderm. In the anterior endoderm, however, suppression of Wnt signaling retains foregut fate and allows subsequent development of the liver (13, 14). Overall, Wnt signaling is tightly regulated during embryo development, which is particularly important at the initial stages of liver development. Absence of Wnt signaling activity will result in impaired hepatic development. This is supported by the elevated expression of Wnt downstream core transcription factors during the terminal differentiation of hepatocytes (15).
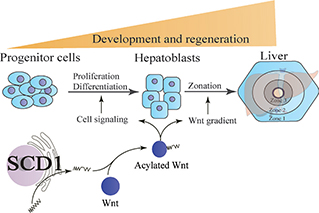
Figure 2. Stearoyl-coenzyme A desaturase 1 (SCD1) contributes to liver development and regeneration by modulating Wnt activity. Microsomal SCD1 produces monounsaturated fatty acids that can be attached to Wnts. This process, termed acylation, enables Wnt secretion and activation. Wnt acylation is also a prerequisite for the formation of concentration and activity gradients of Wnts. The Wnt gradients mediate zonal development of liver and regeneration.
SCD1 shows a determinant role in the in vitro differentiation process of human-induced pluripotent stem cells toward hepatic lineage. Inhibition of SCD1 by a selective inhibitor in early stages of in vitro induced differentiation has resulted in decreased hepatic markers. In the rescue experiments with the combination of the SCD1 inhibitor and its main product oleate, the effect of inhibitor was strongly reversed (16). Our ongoing in vivo work is to identify the role of SCD1 in the early stages of liver organogenesis and development, especially its role in the formation of a complete liver before birth.
Apart from hepatocyte differentiation, Wnt signaling pathway plays a central role in liver zonation (17). Hepatocytes adjacent to portal vein are named periportal hepatocytes (zone 1) and involved in glyconeogenesis and β-oxidation, whereas those near the central vein are called pericentral hepatocytes (zone 3) and play a distinct role in drug metabolism and glycolysis. Hepatocytes in zone 2 exhibit an intermediary role having both periportal and pericentral functions. In a recent study, a high β-catenin-dependent Wnt signaling activity was observed in zone 3 (17–19). These findings collectively highlight the importance of the SCD1-produced MUFAs in mediating liver development through Wnt regulation.
Recent studies using animal models of partial hepatectomy have also implicated the direct involvement of Wnt signaling pathway in liver regeneration. The expression and nuclear translocation of β-catenin significantly increase within minutes of hepatectomy and promote hepatocyte proliferation rate (20). In acetaminophen-induced liver injury, Wnt/β-catenin not only induces the expression of enzymes involved in drug metabolism such as cytochrome P450 2E1 and cytochrome P450 1A2 but also helps liver regeneration (21, 22). In line with this finding, SCD1 gene expression was increased 3.5-fold in regenerating liver following major liver resection (23). After tissue damage, the residual cells start the repair process (24), and the proliferating cells accumulate several categories of lipids including triglycerides, fatty acids (both saturated and unsaturated), and cholesterol esters (25). These lipids are thought to undergo oxidation and provide the required energy for cell proliferation. In addition, they are the main components of newly synthetized membranes. Besides, these lipids can contribute to various signal transduction cascades related to cell proliferation and differentiation such as Wnt and hedgehog signaling pathways (26). It is hypothesized that MUFAs produced by SCD1 not only provide metabolic energy source and structural components, but also promote protein acylation–mediated signaling, which are essential for liver regeneration.
SCD1 Prevents Lipotoxicity and Controls Hepatic Lipogenesis
Palmitic acid and stearic acid are the major de novo synthesized lipotoxic saturated fatty acids (SFAs) in the liver. SCD1 mediates the addition of a double bond to the saturated carbon chain. On one hand, SCD1 activity attenuates SFAs lipotoxic effects through their conversion into the MUFAs palmitoleic acid and oleic acid (27). On the other hand, SCD1 generates unsaturated fatty acids serving as rate-limiting substrates for lipogenesis (28). Thus, an improper increase in its activity may cause hepatic lipid accumulation.
Despite oleate being the major dietary MUFA, SCD1 expression is highly regulated in response to developmental, dietary, environmental, and hormonal factors. De novo synthesized MUFAs are the preferred substrates for neutral hepatic lipid synthesis including triglycerides and cholesterol ester. SCD1 inhibition protects against high-fat high-carbohydrate diet, leptin-deficiency-induced obesity, and hepatic steatosis (29). Leptin-deficient mice exhibit SCD1 overexpression causing palmitoleate and oleate accumulation in liver as fat droplets. Recent studies on high-carbohydrate-fed rats have revealed that deficiency in SCD1 decreased lipid synthesis, elevated fatty acid oxidation and thermogenesis, and insulin susceptibility in different tissues, especially in the liver (30).
Sampath et al. (31) showed that stearate-rich diet causes SCD1 induction and hepatic lipid accumulation in wild-type mice but not in scd1-/- mice. However, in scd1-/- mice, stearate does not induce genes involved in lipogenesis. Additionally, sterol regulatory element-binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor coactivator-1 (PGC-1) transcription factors, which are necessary mediators for pro-lipogenic activities of saturated fatty acids (SFAs), were downregulated in scd1-/- mice. Instead, fatty acid oxidation genes such as carnitine palmitoyltransferase-1 (CPT-1) were induced, resulting in hepatic glycogen depletion. Lui et al. (32), in a study on zinc finger transcription factor knockout mouse model, showed a significant decrease in SCD1 expression and triglyceride accumulation compared to controls. Binding of this transcription factor to SCD1 promoter in hepatocytes was also reported in this study, indicating its critical role in the activation of SCD1 expression.
The role of SCD1 in gut microbiota-dependent hepatic lipogenesis has been studied by Singh et al. (28). They reported that mice with deficient Toll-like receptor-5, which is expressed in gut epithelial cells and plays an important role in microbiota homeostasis, exhibit a microbiota-dependent metabolic syndrome with elevated hepatic lipogenesis, SCD1 expression and activity, and hepatic neutral lipids accumulation with a high oleate and palmitoleate content.
Furthermore, the expression level of SCD1 and fatty acid synthase along with endoplasmic reticulum (ER) stress markers was downregulated in response to the inhibition of poly ADP-ribose polymerase (PARP), which is overexpressed in long-term high-fat high-sucrose diet in mice, indicating the PARP-SCD1 interaction as a major mechanism in the induction of non-alcoholic fatty liver disease (33). The role of SCD1 in an alcoholic fatty liver disease model was studied by Louinis et al. (34). In that study, mice fed with a low-MUFA diet containing 5% ethanol for 10 days and a single ethanol gavage (5 g/kg) developed severe hepatic injury. Liver-specific Scd1-knock-out (SCD1-LKO) mice were resistant to such hepatic injury.
In a recent cross-sectional clinical study, it has been shown that the serum SCD1 activity index is significantly related to the risk of non-alcoholic fatty liver disease in patients with primary dyslipidemia (35). Of course, while interpreting these data, it should be noted that the index of serum SCD1 activity is not solely determined by the liver. In this field, the Ntambi laboratory has recently shown that hepatic triglyceride accumulation in mice may be induced by increased hepatic trafficking of MUFAs originating from non-liver tissues (36). The latter finding further supports the hypothesis that fine-tuning of hepatic SCD1 activity is critical in variable metabolic states.
SCD1 Modulates Hepatic Inflammation and Oxidative Stress
Current evidence indicates the regulatory role of fatty acids in cellular inflammation. SCD1 plays an important role in maintaining the balance between SFAs and MUFAs. Toxic accumulation of SFAs that are reflected as MUFAs/SFAs imbalance leads to activation of oxidative stress imbalance in hepatocytes (37, 38). SFAs mediate cellular inflammatory response through binding to Toll-like receptor-4, CD14, and myeloid differentiation protein-2, causing increased production of bacterial lipopolysaccharides, oxidized phospholipids, and oxidized low-density lipoproteins through intestinal microbiota modification (39). These findings support the hypothesis that SCD1 activity may be protective against SFA-induced oxidative stress and hepatic inflammation. Lu et al. (40) reported that IKK2 (an activator of NFκB) activation can induce hepatic SCD1 overexpression and triglyceride accumulation in mouse. However, such activation decreased the expression of oxidative stress and prevented hepatic inflammation and fibrosis. Paradoxically, there is evidence that increased SCD1 activity may contribute to inflammation and oxidative stress. In the high-carbohydrate or high-sucrose, very-low-fat diet, oleate supplementation leads to decreased hepatic injury and oxidative stress in mice with liver-specific SCD1-LKO (41). A high-fructose diet in female C57BL/6J mice also induced oxidative stress characterized by hepatic SCD1 overexpression and elevation of inducible nitric oxide synthase levels (42). Ochi et al. (43) also showed the association of SCD1 with the development of non-alcoholic steatohepatitis (NASH) under induced stress condition. Indeed, a knockout C57BL/6 mice model with a low expression and activity of SCD1 showed lower hepatic lipid accumulation and steatosis following tunicamycin-induced ER stress than the wild-type mice with ER stress.
Overall, as illustrated in Figure 3, balanced activity of SCD1 is important for stabilizing the ratio of unsaturated to saturated fatty acids. An increase in this ratio can lead to lipid accumulation and a decrease in this ratio is associated with lipotoxicity. A rise in both lipid accumulation and lipotoxicity may lead to hepatic inflammation and oxidative stress.
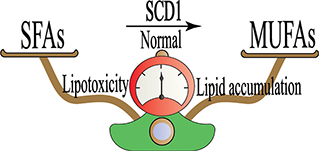
Figure 3. Stearoyl-coenzyme A desaturase 1 (SCD1) activity is associated with normal liver function. The schematic balance represents that the equilibrium between saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) is important in the physiological state. The loss of this equilibrium is due to overactivity and underactivity of SCD1 in hepatic lipid accumulation and lipotoxicity, respectively.
SCD-1 as a Potential Therapeutic Target
The aforementioned studies highlight the significant role of SCD1 activity in hepatic pathophysiology. Therapeutic strategies targeting SCD1 may have applications in managing liver disorders. Several studies have examined small molecule inhibitors in this regard. The following sections review recent advances in small molecule SCD1 inhibitors and their potential therapeutic application in hepatic disorders (Table 1).
Table 1. Stearoyl-coenzyme A desaturase 1 small molecule inhibitors with potential applications for hepatic diseases.
| Compound |
Liver-selective |
Therapeutic application |
Current evidence |
Reference |
| MK-8245 |
+ |
Diabetes, dyslipidemia, HCV |
Preclinical/animal/phase II clinical trial |
(46, 48) |
| Hydroxy pyridone |
+ |
Dyslipidemia- |
In vivo |
(49) |
| N-(2-hydroxy-2-phenylethyl)-6-[4-(2-methylbenzoyl)piperidin-1-yl]pyridazine-3-carboxamide |
- |
Reduction in triglyceride accumulation in NASH |
In vivo |
(50, 51) |
| Thiazole analogs |
- |
HCV infection |
In vitro |
(54) |
| Thiazol-4-acetic acid derivatives |
+ |
Diabetes, hepatic steatosis and obesity |
In vivo |
(55) |
| HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; OATP, organic-anion-transporting polypeptide. |
MK-8245
SCD1 enzyme is expressed in many cell types of the body. Therefore, potential SCD1 inhibitors for the treatment of liver diseases will have a lot of side effects although they might be highly selective toward SCD1. For example, SCD1-knockout rodent models and SCD1 inhibitor–treated rats develop severe skin and eye abnormalities (44, 45). Therefore, liver-specific targeting of SCD1 may be an effective strategy for the treatment of liver-related disorders. One such inhibitor is MK-8245 (46). It has a transporting element that specifically interacts with heptocytes via the liver-specific organic anion transporting polypeptides. Administration of MK-8245 to mice fed with a high-fat diet did not reduce food intake. Despite this, a reduction in liver steatosis and a decrease in liver triglyceride levels were observed. MK-8245 also exhibited anti-diabetic and anti-dyslipidemic properties. Administration of MK-8245 to individuals with type 2 diabetes mellitus in a phase II clinical trial showed no serious adverse events (47). MK-8245 may also be an option for anti-hepatitis C virus (HCV) therapy as evaluated using recombinant HCV culture systems (48). The potential therapeutic effects of this compound on liver diseases are yet to be clinically examined.
Hydroxy pyridone
Another compound that was used for targeted liver SCD1 inhibition is 4-hydroxy pyridine (49). According to the pharmacokinetic analysis, this SCD1 inhibitor had a significantly higher concentration in liver than in plasma and eyelid. It showed a good potency in reducing the mouse liver ratio of palmitoleate to palmitate as a biomarker for SCD1 activity (49).
Pyridazine derivative
A piperazine-based SCD-1 inhibitor, N-(2-hydroxy-2-phenylethyl)-6-[4-(2 methylbenzoyl)piperidin-1-yl]pyridazine-3-carboxamide, has been shown to produce beneficial effects in experimental modes of NASH (50, 51). When administered orally for 8 weeks, once daily, triglyceride accumulation in the liver was reduced by 80% from the fourth week. It also attenuated the increase of aspartate aminotransferase and alanine transaminase by 86% and 78%, respectively. Hepatic steatosis, hepatocellular degeneration, and inflammatory cell infiltration were also ameliorated after the treatment (50).
Thiazole analogs
Current evidence shows that host cell lipid homeostasis plays a critical role in the pathogenesis of HCV by facilitating the formation of viral membrane-associated replication complex. Since inhibiting lipogenesis has a negative effect on virus proliferation, inhibiting the lipid synthesis enzyme SCD1 is a potential strategy for HCV treatment (52, 53). Lyn et al. (54) showed that the thiazole compound MF-152 can repress HCV infection in human hepatoma cells by modifying membrane functions which are required for HCV replication. Cell imaging studies showed that the inability of viral RNAs to interact with modified membranes exposes them to degradation by endogenous nucleases. This process ultimately prevents the formation of HCV viral complexes and arrests HCV replication.
Thiazole-4-acetic acid derivative
Another potent liver-selective SCD1 inhibitor, compound 48 (a thiazole-4-acetic acid derivative), has recently been discovered through high-throughput screening efforts following an ex vivo assay approach on mice liver and eyelids (55). Administration of compound 48 to mice fed a high-fat diet for 43 days improved glucose tolerance and decreased body weight without adverse effects on skin or eyes. Furthermore, compound 48 significantly attenuated hepatic triglyceride accumulation in rats fed a high-sucrose, very-low-fat diet (55). These findings suggest that compound 48 may have clinical benefits in the treatment of diabetes, hepatic steatosis, and obesity through targeting SCD1 activity in liver.
Conclusion
SCD1, one of the predominantly expressed enzymes in the liver, is a major factor in fatty acid metabolism. It plays a regulatory role in posttranslational protein modifications via protein acylation. Wnts play an important role in hepatic differentiation, zonation, and regeneration. The Wnts pathway, at least in part, is regulated by SCD1-mediated palmitoleoylation and oleoylation. In addition, SCD1 is crucial for SFA detoxification and MUFA production. SFAs overproduction induces hepatic inflammation and oxidative stress, and SCD1 attenuates these effects via monounsaturation of SFAs. Meanwhile, de novo produced MUFAs promote and participate in cellular lipogenesis and their overproduction can lead to hepatic lipid accumulation. Targeting SCD1 as a novel therapeutic approach may be beneficial in liver disorders. In this regard, several studies have tested small molecule inhibitors of SCD1 in vitro and in vivo, which opens a promising point of view in the treatment of liver metabolic diseases.
Acknowledgments
This study was supported by a grant (No. 5.4.9705) from the Emergency Medicine Research Team at Tabriz University of Medical Sciences.
Conflict of interest
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- Ntambi JM, Buhrow SA, Kaestner KH, Christy RJ, Sibley E, Kelly TJ, et al. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem [Internet]. 1988 Nov 25;263(33):17291–300. Available from (accessed 25.12.18.): http://www.ncbi.nlm.nih.gov/pubmed/2903162
- Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008 Jun;19(3):248–56. https://doi.org/10.1097/MOL.0b013e3282f9b54d
- Arregui M, Buijsse B, Stefan N, Corella D, Fisher E, di Giuseppe R, et al. Heterogeneity of the Stearoyl-CoA desaturase-1 (SCD1) gene and metabolic risk factors in the EPIC-Potsdam study. PLoS One. 2012;7:e48338. https://doi.org/10.1371/journal.pone.0048338
- Mauvoisin D, Charfi C, Lounis AM, Rassart E, Mounier C. Decreasing stearoyl-CoA desaturase-1 expression inhibits β-catenin signaling in breast cancer cells. Cancer Sci. 2013 Jan;104(1):36–42. Available from: https://doi.org/10.1111/cas.12032
- Zhang L, Ge L, Tran T, Stenn K, Prouty SM. Isolation and characterization of the human stearoyl-CoA desaturase gene promoter: Requirement of a conserved CCAAT cis-element. Biochem J. 2001;357:183–93. https://doi.org/10.1042/bj3570183
- Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, et al. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015 Aug;524(7564):252–6. Available from: https://doi.org/10.1038/nature14549
- Mehdizadeh A, Fayezi S, Darabi M. SCD (stearoyl-CoA desaturase (delta-9-desaturase)). Atlas Genet Cytogenet Oncol Haematol. 2016;20(2):77–80. https://doi.org/10.4267/2042/62515
- Kim YC, Gomez FE, Fox BG, Ntambi JM. Differential regulation of the stearoyl-CoA desaturase genes by thiazolidinediones in 3T3-L1 adipocytes. J Lipid Res [Internet]. 2000 Aug;41(8):1310–16. Available from (accessed 25.12.18.): http://www.ncbi.nlm.nih.gov/pubmed/10946019
- Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim H-J, et al. Stearoyl-CoA Desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004 Jun;279(24):25164–71. https://doi.org/10.1074/jbc.M402781200
- Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145(11):1–8. https://doi.org/10.1242/dev.146589
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012 Jul;337(6090):59–64. https://doi.org/10.1126/science.1222879
- Sekine K, Takebe T, Taniguchi H. Liver regeneration using cultured liver bud. Methods Mol Biol. 2017;1597:207–16. https://doi.org/10.1007/978-1-4939-6949-4_15
- Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: Cell signaling and transcriptional control. Genes Dev. 1996 Jul;10(13):1670–82. https://doi.org/10.1101/gad.10.13.1670
- Lokmane L, Haumaitre C, Garcia-Villalba P, Anselme I, Schneider-Maunoury S, Cereghini S. Crucial role of vHNF1 in vertebrate hepatic specification. Development. 2008 Aug;135(16):2777–86. https://doi.org/10.1242/dev.023010
- Lemaigre FP. Mechanisms of liver development: Concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009 Jul;137(1):62–79. https://doi.org/10.1053/j.gastro.2009.03.035
- Rahimi Y, Mehdizadeh A, Nozad Charoudeh H, Nouri M, Valaei K, Fayezi S, et al. Hepatocyte differentiation of human induced pluripotent stem cells is modulated by stearoyl-CoA desaturase 1 activity. Dev Growth Differ. 2015 Dec;57(9):667–74. Available from: https://doi.org/10.1111/dgd.12255
- Schröder E, Höhme S, Böttger J, Aleithe S, Gebhardt R, Matz-Soja M. Zonation of Morphogens in the adult liver – Crosstalk between Hh and Wnt/β-Catenin signaling. Z Gastroenterol. 2018 Jan;56(01):E2–89. https://doi.org/10.1055/s-0037-1612748
- Brosch M, Kattler K, Herrmann A, von Schönfels W, Nordström K, Seehofer D, et al. Epigenomic map of human liver reveals principles of zonated morphogenic and metabolic control. Nat Commun. 2018 Oct;9(1):4150. https://doi.org/10.1038/s41467-018-06611-5
- Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: A sequel to the Wnt-Wnt situation. Hepatol Commun. 2018 Jul;2(7):845–60. https://doi.org/10.1002/hep4.1196
- Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001 Dec;61(23):8564–8.
- Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/β-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011 Apr;30(16):1868–79. https://doi.org/10.1038/onc.2010.560
- Dadhania VP, Bhushan B, Apte U, Mehendale HM. Wnt/β-Catenin signaling drives thioacetamide-mediated heteroprotection against acetaminophen-induced lethal liver injury. Dose Response. 15(1):1559325817690287. https://doi.org/10.1177/1559325817690287
- Xu C, Lin F, Qin S. Relevance between lipid metabolism-associated genes and rat liver regeneration. Hepatol Res. 2008 Aug;38(8):825–37. https://doi.org/10.1111/j.1872-034X.2008.00345.x
- Forbes SJ, Newsome PN. Liver regeneration—mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13(8):473–85. https://doi.org/10.1038/nrgastro.2016.97
- Bellet MM, Masri S, Astarita G, Sassone-Corsi P, Della Fazia MA, Servillo G. Histone deacetylase SIRT1 controls proliferation, circadian rhythm, and lipid metabolism during liver regeneration in mice. J Biol Chem. 2016;291(44):23318–29. https://doi.org/10.1074/jbc.M116.737114
- Kohjima M, Tsai T-H, Tackett BC, Thevananther S, Li L, Chang BH-J, et al. Delayed liver regeneration after partial hepatectomy in adipose differentiation related protein-null mice. J Hepatol. 2013 Dec;59(6):1246–54. https://doi.org/10.1016/j.jhep.2013.07.025
- Collins JM, Neville MJ, Hoppa MB, Frayn KN. De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. J Biol Chem. 2010;285:6044–52. https://doi.org/10.1074/jbc.M109.053280
- Singh V, Chassaing B, Zhang L, San Yeoh B, Xiao X, Kumar M, et al. Microbiota-dependent hepatic lipogenesis mediated by Stearoyl CoA Desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 2015 Dec;22(6):983–96. https://doi.org/10.1016/j.cmet.2015.09.028
- Miyazaki M, Sampath H, Liu X, Flowers MT, Chu K, Dobrzyn A, et al. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem Biophys Res Commun. 2009 Mar;380(4):818–22. https://doi.org/10.1016/j.bbrc.2009.01.183
- Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007 Dec;6(6):484–96. https://doi.org/10.1016/j.cmet.2007.10.014
- Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007 Jan;282(4):2483–93. Available from: https://doi.org/10.1074/jbc.M610158200
- Liu G, Zhou L, Zhang H, Chen R, Zhang Y, Li L, et al. Regulation of hepatic lipogenesis by the zinc finger protein Zbtb20. Nat Commun. 2017 Mar;8:14824. https://doi.org/10.1038/ncomms14824
- Gariani K, Ryu D, Menzies KJ, Yi H-S, Stein S, Zhang H, et al. Inhibiting poly ADP-ribosylation increases fatty acid oxidation and protects against fatty liver disease. J Hepatol. 2017;66(1):132–41. https://doi.org/10.1016/j.jhep.2016.08.024
- Lounis MA, Escoula Q, Veillette C, Bergeron K-F, Ntambi JM, Mounier C. SCD1 deficiency protects mice against ethanol-induced liver injury. Biochim Biophys Acta. 2016;1861(11):1662–70. https://doi.org/10.1016/j.bbalip.2016.07.012
- Amor AJ, Cofán M, Mateo-Gallego R, Cenarro A, Civeira F, Ortega E, et al. Dietary polyunsaturated fatty acids mediate the inverse association of stearoyl-CoA desaturase activity with the risk of fatty liver in dyslipidaemic individuals. Eur J Nutr. 2018 Apr; Available from: https://doi.org/10.1007/s00394-018-1691-4
- Bond LM, Ntambi JM. UCP1 deficiency increases adipose tissue monounsaturated fatty acid synthesis and trafficking to the liver. J Lipid Res. 2018 Feb;59(2):224–36. Available from: https://doi.org/10.1194/jlr.M078469
- Wek RC, Anthony TG. Obesity: Stressing about unfolded proteins. Nat Med. 2010 Apr;16(4):374–6. https://doi.org/10.1038/nm0410-374
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007 Jul;8(7):519–29. https://doi.org/10.1038/nrm2199
- Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016 Jan;244:211–5. https://doi.org/10.1016/j.atherosclerosis.2015.11.015
- Lu H, Lei X, Zhang Q. Moderate activation of IKK2-NF-kB in unstressed adult mouse liver induces cytoprotective genes and lipogenesis without apparent signs of inflammation or fibrosis. BMC Gastroenterol. 2015 Jul;15:94. https://doi.org/10.1186/s12876-015-0325-z
- Liu X, Burhans MS, Flowers MT, Ntambi JM. Hepatic oleate regulates liver stress response partially through PGC-1α during high-carbohydrate feeding. J Hepatol. 2016;65(1):103–12. https://doi.org/10.1016/j.jhep.2016.03.001
- Choi Y, Abdelmegeed MA, Song B-J. Diet high in fructose promotes liver steatosis and hepatocyte apoptosis in C57BL/6J female mice: Role of disturbed lipid homeostasis and increased oxidative stress. Food Chem Toxicol. 2017 May;103:111–21. https://doi.org/10.1016/j.fct.2017.02.039
- Ochi T, Munekage K, Ono M, Higuchi T, Tsuda M, Hayashi Y, et al. Patatin-like phospholipase domain-containing protein 3 is involved in hepatic fatty acid and triglyceride metabolism through X-box binding protein 1 and modulation of endoplasmic reticulum stress in mice. Hepatol Res. 2016 May;46(6):584–92. https://doi.org/10.1111/hepr.12587
- Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001 Sep;131(9):2260–8. Available from: https://doi.org/10.1093/jn/131.9.2260
- Ramtohul YK, Black C, Chan C-C, Crane S, Guay J, Guiral S, et al. SAR and optimization of thiazole analogs as potent stearoyl-CoA desaturase inhibitors. Bioorg Med Chem Lett. 2010 Mar;20(5):1593–7. https://doi.org/10.1016/j.bmcl.2010.01.083
- Oballa RM, Belair L, Black WC, Bleasby K, Chan CC, Desroches C, et al. Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia. J Med Chem. 2011 Jul;54(14):5082–96. https://doi.org/10.1021/jm200319u
- Merck Sharp & Dohme Corp. A study to assess the safety and efficacy of MK8245 in patients with type 2 diabetes mellitus and inadequate glycemic control (MK8245-005 AM2) [Internet]. ClinicalTrials.gov Web Site. 2019. Available from (accessed 25.12.18.): https://clinicaltrials.gov/ct2/show/study/NCT00846391
- Nio Y, Hasegawa H, Okamura H, Miyayama Y, Akahori Y, Hijikata M. Liver-specific mono-unsaturated fatty acid synthase-1 inhibitor for anti-hepatitis C treatment. Antiviral Res. 2016 Aug;132:262–7. Available from: https://doi.org/10.1016/j.antiviral.2016.07.003
- Sun S, Zhang Z, Raina V, Pokrovskaia N, Hou D, Namdari R, et al. Discovery of thiazolylpyridinone SCD1 inhibitors with preferential liver distribution and reduced mechanism-based adverse effects. Bioorg Med Chem Lett. 2014 Jan;24(2):526–31. https://doi.org/10.1016/j.bmcl.2013.12.035
- Uto Y, Ogata T, Kiyotsuka Y, Ueno Y, Miyazawa Y, Kurata H, et al. Novel benzoylpiperidine-based stearoyl-CoA desaturase-1 inhibitors: Identification of 6-[4-(2-methylbenzoyl)piperidin-1-yl]pyridazine-3-carboxylic acid (2-hydroxy-2-pyridin-3-ylethyl)amide and its plasma triglyceride-lowering effects in Zucker fatty rats. Bioorg Med Chem Lett. 2010 Jan;20(1):341–5. https://doi.org/10.1016/j.bmcl.2009.10.101
- Kurikawa N, Takagi T, Wakimoto S, Uto Y, Terashima H, Kono K, et al. A novel inhibitor of stearoyl-CoA desaturase-1 attenuates hepatic lipid accumulation, liver injury and inflammation in model of nonalcoholic steatohepatitis. Biol Pharm Bull. 2013;36(2):259–67. https://doi.org/10.1248/bpb.b12-00702
- Pezacki JP, Singaravelu R, Lyn RK. Host-virus interactions during hepatitis C virus infection: A complex and dynamic molecular biosystem. Mol Biosyst. 2010 Jul;6(7):1131–42. https://doi.org/10.1039/b924668c
- Herker E, Ott M. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol Metab. 2011 Jun;22(6):241–8. Available from: https://doi.org/10.1016/j.tem.2011.03.004
- Lyn RK, Singaravelu R, Kargman S, O’Hara S, Chan H, Oballa R, et al. Stearoyl-CoA desaturase inhibition blocks formation of hepatitis C virus-induced specialized membranes. Sci Rep. 2014 Apr;4:4549. https://doi.org/10.1038/srep04549
- Iida T, Ubukata M, Mitani I, Nakagawa Y, Maeda K, Imai H, et al. Discovery of potent liver-selective stearoyl-CoA desaturase-1 (SCD1) inhibitors, thiazole-4-acetic acid derivatives, for the treatment of diabetes, hepatic steatosis, and obesity. Eur J Med Chem. 2018 Oct;158:832–52. https://doi.org/10.1016/j.ejmech.2018.09.003