REVIEW ARTICLE
Anaemia of Chronic Kidney Disease: What We Know Now
Anoushka R. Krishnan1, Debbie Trinder2,3,4, Anita C. G. Chua2,3,4, Aron Chakera1, Grant A. Ramm5,6, John K. Olynyk2,3,7,8,9,10
1Department of Nephrology, Sir Charles Gairdner Hospital, Perth, Western Australia, Australia; 2School of Medicine and Pharmacology, University of Western Australia, Perth, Western Australia, Australia; 3Fiona Stanley Hospital, Murdoch, Western Australia, Australia; 4Harry Perkins Institute of Medical Research, Murdoch, Western Australia, Australia; 5QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia; 6Faculty of Medicine and Biomedical Sciences, The University of Queensland, Brisbane, Queensland, Australia; 7Department of Gastroenterology, Fiona Stanley Hospital, Perth, Western Australia, Australia; 8Faculty of Health Sciences, Edith Cowan University, Joondalup, Western Australia, Australia; 9School of Biomedical Sciences, Curtin University, Bentley, Western Australia, Australia; 10School of Veterinary and Life Sciences, Murdoch University, Murdoch, Western Australia, Australia
Abstract
Our understanding of the pathophysiology of the anaemia of chronic kidney disease (CKD) has improved considerably in the last decade with the discovery of the iron regulatory peptide hepcidin. Reduced clearance of hepcidin and the presence of a chronic inflammatory state contribute to elevated hepcidin levels in kidney disease. The recent discovery of the various factors and signalling pathways regulating hepcidin has opened up an exciting avenue for research into the development of newer agents that could treat anaemia of CKD. This review highlights our current understanding of iron metabolism in health, the regulators of hepcidin, issues associated with the current available therapies for the treatment of anaemia in CKD and potential novel therapies that could be available in the near future targeting the various factors that regulate hepcidin.
Keywords: anaemia; chronic kidney disease; iron metabolism; hepcidin; inflammation; erythropoietin-stimulating agents
Received: 28 November 2016;
Accepted after revision: 02 January 2017;
Published: 03 February 2017.
Author for correspondence: John K. Olynyk, Fiona Stanley Hospital, 11, Robin Warren Drive, Murdoch, Western Australia, Australia 6150, Postal address: Locked Bag 100, PALMYRA DC, WA 6961. Email:
john.olynyk@health.wa.gov.auHow to cite: Krishnan AR et al. Anaemia of chronic kidney disease: what we know now. J Ren Hepat Disord 2017;1(1):11–19.
DOI:
http://dx.doi.org/10.15586/jrenhep.2017.5Copyright: Krishnan AR et al.
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
Anaemia of chronic kidney disease (CKD) is widely prevalent in patients with renal impairment and is associated with significant morbidity and mortality (1, 2). Deficient erythropoietin (EPO) production and reduced bioavailability of iron ultimately lead to absolute or functional iron deficiency anaemia. Hepcidin, an iron regulatory protein produced in the liver by hepatocytes, plays an important role in iron metabolism by regulating iron absorption from the duodenum and iron release from macrophages by interacting with, and inactivating, ferroportin—the iron transport protein (3). Hepcidin is regulated by a number of factors including iron status, inflammation, erythropoiesis and hypoxia, which are often affected by kidney disease.
Iron Metabolism
Iron is an essential trace element required for a number of catabolic and metabolic processes within the body. As there are no effective means of excreting iron, the regulation of dietary iron absorption from the duodenum plays an important role in iron homeostasis. In healthy individuals, approximately 1–2 mg of iron is absorbed from the diet per day to maintain iron balance. Once absorbed, the iron is bound by the plasma protein transferrin and is transported to the tissues where most of the iron is taken up by the bone marrow for incorporation into haemoglobin for erythropoiesis and to a lesser degree by the muscle for the synthesis of myoglobin and respiratory enzymes. Excess iron is stored primarily in the liver. Macrophages degrade erythrocyte-derived haemoglobin and release the iron back into the plasma so that it can be re-utilised for erythropoiesis in the bone marrow. If the availability of iron for erythropoiesis is insufficient, anaemia will develop. Too much iron can result in iron overload. Common causes include genetic diseases such as hereditary haemochromatosis and acquired causes such as from transfusional overload and repeated parenteral iron infusions. Iron excess is detrimental to health as this generates free radicals causing oxidative stress and tissue damage primarily in the liver, heart and pancreas (4–8).
Hepcidin and Its Regulators
Iron metabolism is tightly regulated by the hormone hepcidin which is highly expressed by hepatocytes and at lower levels in other tissues including the kidneys (9). Hepcidin, a 25-amino acid cysteine-rich peptide, is a negative regulator of iron absorption by the intestine and iron release from macrophages and hepatic stores. It is secreted into the circulation and binds to the iron exporter ferroportin, expressed on the surface of enterocytes, macrophages and hepatocytes, causing ferroportin internalisation and degradation. This limits the absorption and release of iron and increases retention in the liver and macrophages (6, 10).
In addition to regulating iron metabolism, hepcidin may also contribute indirectly to host defence mechanisms by reducing body iron concentrations, as iron is needed for bacterial growth and low levels of iron are thought to be bacteriostatic. In murine models and cultured macrophages, hepcidin has been found to modulate lipopolysaccharide-induced transcription, suggesting it might have a role in modulating acute inflammatory responses to bacterial infections (11, 12).
The two main positive regulators of hepcidin are iron status and inflammation with higher levels limiting the availability of iron for erythropoiesis and other iron-dependent processes. Similarly, erythropoiesis and hypoxia downregulate hepcidin expression, resulting in increased bioavailability of iron (Figure 1). These factors regulate hepcidin levels via pathways listed in Table 1, and some of these pathways could be potential targets for novel therapies to treat anaemia of CKD.
Table 1. Regulation of hepcidin
| Regulators |
Signalling pathway |
| Iron status |
BMP6/SMAD and HFE/TFR2 |
| Inflammation (interleukin-6) |
JAK2/STAT3, activin B |
| Hypoxia |
HIFs and EPO |
| Erythropoiesis |
EPO and ERFE |
| BMP6: bone morphogenic protein 6; SMAD: mothers against decapentaplegic-related protein; HFE/TFR2: haemochromatosis iron protein/transferrin receptor 2; JAK: Janus kinase; STAT3: signal transducer and activation of transcription 3; HIFs: hypoxia-inducible factors; EPO: erythropoietin; ERFE: erythroferrone. |
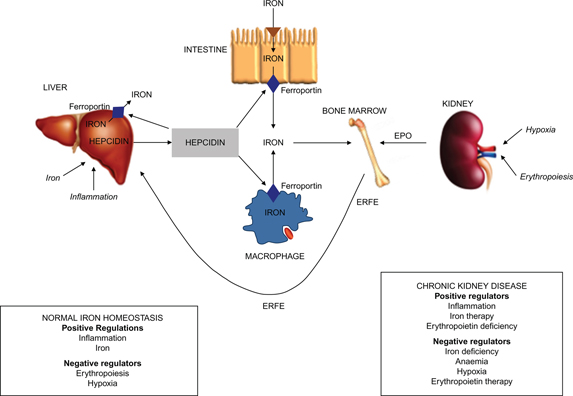
Figure 1. Schematic representation of the role of hepcidin in iron metabolism in health and in chronic kidney disease (CKD). Both iron and erythropoietin (EPO) are required for the production of red blood cells in the bone marrow. Hepcidin regulates iron absorption from the intestine, macrophage iron recycling from senescent red blood cells and iron release from the liver via ferroportin, the iron transport protein. Hepcidin causes degradation of ferroportin leading to cellular iron retention and decreased absorption of ingested iron. Several factors including iron and inflammation act directly on the liver to up-regulate hepcidin production. Erythropoiesis and hypoxia negatively regulate hepcidin production indirectly, by increasing EPO production by the kidneys. This stimulates synthesis of erythroferrone (ERFE) by the bone marrow, which in turn controls liver hepcidin production. In CKD, anaemia occurs due to reduced EPO production from the kidneys and from reduced iron absorption and availability, the latter resulting from elevated levels of hepcidin. In CKD, hepcidin levels are raised due to the combination of increased inflammation, decreased EPO levels and reduced renal clearance. EPO therapy decreases hepcidin levels leading to iron mobilisation from body stores during erythropoiesis.
Regulation of Hepcidin
Iron status
Tissue iron stores and circulating transferrin-bound iron exert distinct signals that regulate hepcidin expression in hepatocytes. Hepcidin gene transcription is stimulated by the dual effect of liver iron stores and the concentration of plasma holotransferrin (iron-saturated transferrin), conveyed through iron-regulated production of bone morphogenetic proteins (BMP) acting on BMP receptors and the associated mothers against decapentaplegic-related protein (SMAD) pathway (13). Intracellular iron stores interact with hepcidin via the BMP6 pathway activating SMAD and increasing hepcidin levels. Circulating transferrin-bound iron exerts its effects via the haemochromatosis protein (HFE)/transferrin receptor 2 (TFR2) pathway (14, 15). Mutations of these receptors are associated with hereditary haemochromatosis resulting in iron overload via dysregulated hepcidin expression (16, 17).
Inflammation
Hepcidin levels are increased by states of inflammation, and this is thought to have evolved as a host defence mechanism. Interleukin-6, acting through the JAK2/STAT3 pathway and, to a lesser extent, interferon γ, and interleukin-1 are the primary inflammatory inducers of hepcidin expression (18, 19). Recently, a new inflammatory signalling pathway was identified, stimulating hepcidin production via activin B, BMP receptors and SMAD (20).
Hypoxia
This is a potent inhibitor of hepcidin production, even in the absence of anaemia, and thus increases iron availability (18). Hypoxia-inducible factors (HIFs) are transcription factors that regulate expression of genes in response to hypoxia including genes required for iron metabolism and erythropoiesis. EPO synthesis is regulated in the liver and kidney via HIF-2α. HIF activity is controlled by prolyl-4-hydroxylase domains (PHD), which act as oxygen sensors. At normal oxygen concentrations, PHD enzymes hydroxylate the HIF-α subunit resulting in its rapid degradation. At lower concentrations of oxygen, HIF–PH activity is reduced, and there is accumulation of HIF-α, leading to increased levels of EPO and its receptor, and decreased hepcidin levels, ultimately increasing iron availability and erythropoiesis (21–24). Similar effects were seen during hypoxia at high altitude. In a study of healthy volunteers who were exposed to high altitude levels (3400–5400 m above sea level), hypoxia induced a marked suppression of hepcidin, which appeared to result from the combined action of hypoxia-induced increased erythropoiesis and iron depletion (25).
Erythropoiesis
Increased erythropoiesis appears to suppress hepcidin levels, allowing for higher iron bioavailability to meet increased demands for red blood cell production in such states. Erythroferrone (ERFE), a relatively new hormone identified in 2014, was found to regulate iron metabolism by decreasing hepcidin levels during periods of stress erythropoiesis (26). This protein is thought to be the long-sought erythroid factor that inhibits hepcidin during increased erythropoietic activity and may contribute to the pathogenesis of iron-loading anaemias. Kautz et al. showed in animal models that bleeding or administration of EPO leads to release of ERFE from erythroblasts, which acts directly on hepatocytes to suppress hepcidin (26). In ineffective erythropoiesis, ERFE secreted by the massively increased numbers of erythroid precursors may overwhelm the iron storage signal and shut off hepcidin production (26, 27). Erythropoietin-stimulating agents (ESAs) are widely used to treat anaemia of CKD. ESAs significantly suppress levels of serum hepcidin and ferritin, resulting in effective erythropoiesis and release of stored iron (28, 29). Honda et al. examined the association between ERFE and biomarkers of iron metabolism in haemodialysis patients and found that levels of ERFE were inversely correlated with levels of hepcidin and ferritin and positively correlated with soluble transferrin receptor. They also showed that the use of ESAs increased the levels of ERFE that regulated hepcidin and led to iron mobilisation from body stores during erythropoiesis (30). Additional studies of this pathway and its potential effects in CKD are warranted.
Anaemia of CKD
Anaemia is a common feature of CKD, which increases in prevalence as the severity of CKD progresses. Anaemia in patients with renal failure is associated with poor quality of life and high morbidity rates as evidenced by increased hospitalisations and incidence of cardiovascular disease incorporating left ventricular hypertrophy, heart failure and higher rates of mortality from adverse cardiac events (31, 32).
Anaemia of CKD is typically normochromic and normocytic and is thought to result from two main mechanisms—deficient production of EPO by the kidney and reduced iron absorption and availability. In CKD, iron deficiency can be classified into absolute iron deficiency (marked by low iron stores and circulating iron concentrations) and functional iron deficiency (marked by low circulating iron in the setting of normal iron stores), with both forms leading to iron-restricted erythropoiesis that leads to anaemia of CKD as well as ESA hyporesponsiveness (33).
The reduced absorption and bioavailability of iron is thought to result from excessive production of hepcidin (34, 35), partly contributed by reduced renal clearance (36–38) and partly in response to elevated interleukin-6 or other pro-inflammatory cytokines produced in CKD (6, 39, 40). CKD is associated with a chronic inflammatory state, in particular, elevated interleukin-6 plasma levels, which are a major mediator of the acute-phase response. In In CKD patients, higher levels of interleukin-6 may be related to loss of kidney function, uraemia and its sequelae (such as fluid overload and susceptibility to infections) and possibly dialysis related factors. (41). Excess levels of hepcidin contribute to impaired dietary iron absorption and iron release from body stores (37, 42, 43). Reduced iron availability occurs due to retention of iron in macrophages and hepatocytes, thus elevating iron stores but reducing serum iron and transferrin saturation levels (functional iron deficiency), causing anaemia even in the presence of adequate iron reserves, in contrast to true iron deficiency.
Additional mechanisms have been suggested to contribute to the pathogenesis of anaemia of CKD, including shortened red blood cell lifespan, nutritional deficiencies (folate and B12) due to anorexia, loss via dialysis and increased iron losses (due to uraemia-related platelet dysfunction causing subclinical blood loss, frequent phlebotomy and trapping of blood in dialysis circuits) (44, 45).
More recently, there has been an interest in vitamin D and its associations with anaemia. Initially, this was attributed to the anti-inflammatory and pro-erythropoietic effects of vitamin D (46). Data now suggest that vitamin D may actually modulate iron homeostasis via hepcidin, with a study showing that 1,25-dihydroxycholecalciferol directly inhibits hepcidin expression by binding to a vitamin D response element in the gene coding for hepcidin (47).
Current Management of Anaemia of CKD
Intravenous iron therapy and ESAs are the cornerstones of current therapy for anaemia related to CKD; however, they are not without their side effects.
Issues with iron therapy
With regard to iron administration during episodes of acute infection or inflammation which results in elevated serum ferritin levels, opinions suggest that iron therapy should be withheld under such circumstances, citing a concern that iron may further help in the proliferation of microorganisms. Iron loading has been shown to be associated with worse outcomes in infectious diseases such as malaria, tuberculosis and HIV (48–50). However, surprisingly, the recent intravenous iron or placebo for anaemia in the intensive care unit (IRONMAN) clinical trial demonstrated no adverse effects of iron administration in acutely unwell intensive care patients but there were significant improvements in haemoglobin levels (51).
Moreover, there are concerns related to iron therapy-induced iron overload, with a study by Barany et al. suggesting that haemodialysis patients with very high ferritin levels have a mean liver iron concentration similar to that of patients with untreated idiopathic haemochromatosis (52). Iron deposition has been associated with the pathogenesis of several other disorders such as diabetes mellitus, neurodegenerative diseases and atherosclerosis (53–55).
Iron could be a potential link between oxidative stress and cardiovascular disease (56, 57). Iron has been found in advanced human atherosclerotic plaques (58), and free iron may play a role in plaque destabilisation post intra-plaque haemorrhage (59), although, despite pathogenic hypotheses, hard evidence linking iron, oxidative stress and cardiovascular disease is limited (60). Also, the long-term effects of high-dose iron therapy remain unclear.
Large prospective randomised controlled trials in the CKD population are long overdue to assess the efficacy of recurrent iron infusions with regard to long-term safety, mortality and morbidity. In the absence of clear target values for serum ferritin and transferrin, clinicians continue to make a case-by-case decision on the best treatment option for their patients.
Advent of ESAs
The treatment of anaemia of CKD was revolutionised in the 1980s with the development of recombinant ESAs, which has reduced the need for blood transfusions (which in turn reduces the chances of acquiring blood-borne infection and avoids sensitisation in potential renal transplant candidates) (61, 62) and improves exercise tolerance, quality of life symptoms and left ventricular hypertrophy (63, 64).
Risk profile of ESAs
The target haemoglobin level in the treatment of CKD has been debated for some time, and a number of clinical trials have sought to assess whether full correction of anaemia to normal levels confers benefits; however, results have been disappointing. In 2006, two large randomised control trials in CKD showed that complete correction of anaemia had no effect (65) or conferred a greater risk for attaining the primary composite cardiovascular endpoint (66). The Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT)-diabetic patients showed no survival benefit in patients when a haemoglobin target of 13 g/l was set and a secondary analysis suggested higher risk for stroke, death resulting from cancer in patients with a history of malignancies, and venous and arterial thromboembolic events (67, 68).
Another limitation of ESAs is that they also necessitate regular injections, either subcutaneous for patients with CKD and on peritoneal dialysis or via the intravenous route for patients on haemodialysis. Supra-physiologic effects of ESAs, especially at high doses, have off-target effects on other cell types expressing EPO receptors including endothelial cells. This results in adverse effects such as hypertension (69), intimal hyperplasia especially in the setting of inflammation (70) and promotion of tumour growth (71). Other drawbacks include the development of ESA hyporesponsiveness (which can occur in 10%–20% of patients with end-stage kidney disease). Patients needing greater doses are those with concomitant infectious, inflammatory or malignant conditions resulting in relative ESA resistance, which may contribute to increased mortality (72).
Given the high costs and potential disadvantages of ESAs, further elucidation of the molecular mechanisms of anaemia in CKD and the development of better targeted therapies have become a priority.
The Hunt for New Therapies
Pentoxifylline
Given that inflammation contributes to elevated hepcidin levels and may contribute to ESA hyporesponsiveness, it was thought that pentoxifylline might partially correct pro-inflammatory cytokines levels in CKD, resulting in improved iron utilisation and erythropoiesis. The drug has been shown to have anti-inflammatory properties (anti-apoptosis, anti-oxidant, anti-TNF-α and anti-IFN-γ) (73–75). In the handling erythopoietin resistance with oxpentifylline (HERO) trial, which was a double-blind, randomised, placebo-controlled trial, Johnson et al. studied the effects of pentoxifylline on ESA hyporesponsive anaemia in 53 patients with CKD stage 4 or 5 (including dialysis) (76). Although pentoxifylline did not significantly modify ESA hyporesponsiveness as measured by the erythropoiesis resistance index, it did safely increase mean haemoglobin concentration significantly, relative to the control group. A smaller sub-study of the HERO trial examined the effect of pentoxifylline on serum hepcidin level but found no significant difference in patients who received the drug as compared to those on placebo (77). A small uncontrolled pilot study that looked at the effect of this drug on inflammation showed that pentoxifylline reduced levels of interleukin-6 and improved haemoglobin levels in non-inflammatory moderate to severe CKD (78). A systematic review and meta-analysis of 11 studies did not demonstrate conclusive effects of pentoxifylline on haematocrit and ESA dosing (79). Whether this drug may provide any benefits remains to be seen in larger randomised trials.
HIF–PH inhibitors
The role of hypoxia in hepcidin regulation has been briefly explained above. Small-molecule inhibitors of the PHD enzymes mimic the response to a cellular reduction in oxygen levels, increase HIF levels and thereby increase EPO production, thus promoting erythropoiesis. These drugs are in various phases of clinical development for the treatment of renal anaemia.
One such agent roxadustat, an oral HIF–PH inhibitor, was trialled in 60 incident dialysis patients in a phase 2 clinical trial and was shown to increase haemoglobin levels by ≥2.0 g/l within 7 weeks, regardless of baseline iron repletion status, C-reactive protein levels, iron regimen or dialysis modality. It was also found to reduce serum hepcidin levels. Roxadustat by inhibiting HIF–PHs results in increased levels of HIF and stimulates erythropoiesis (80).
More recently, Pergola et al. studied the effect of vadadustat as compared to placebo in 210 non-dialysis-dependent CKD patients (stages 3–5) in a 20-week multi-centre phase 2b study (81). They showed that 55% of the candidates who received vadadustat achieved the primary end point (percentage of participants who during the last 2 weeks of the treatment achieved or maintained a mean haemoglobin level of >11 g/dl or an increase in haemoglobin level of ≥1.2 g/dl over the pre-dose average) as compared to 10% of the placebo-treated candidates, and the drug raised and maintained haemoglobin in a predictable manner with no significant side effects as compared to placebo. They noted significant increases in both reticulocyte and total iron-binding capacity and significant decreases in both serum hepcidin and ferritin levels.
Similar small preclinical and clinical studies have demonstrated some pleiotropic effects of this class of drug (molidustat corrected anaemia in rat studies and also helped normalise blood pressure; daprodustat was shown to improve cholesterol levels) (82, 83). Advantages of this new class of drug include oral administration, low immunogenicity, product stability and possibly lower costs as well as potential cardiovascular benefits (84).
Safety concerns of targeting the HIF pathway include the potential for raised Vascular endothelial growth factor (VEGF) production, which is a HIF-related angiogenic growth factor known to be associated with vasculopathies and progression of tumour growth (85), and the potential for development of pulmonary and systemic hypertension, given the role of HIF in regulation of vascular tone (86, 87). Results of phase 3 trials that continue to monitor for these side effects are awaited.
Hepcidin antagonists
Several agents that can antagonise hepcidin are under development, including neutralising hepcidin peptide by anti-hepcidin antibodies or by engineered hepcidin binders such as anticalins (engineered human proteins that can bind specific target molecules). Cooke et al. demonstrated an increase in haemoglobin by 1.5 g/dl within 1 week of injection of an anti-hepcidin antibody in humanised murine models where endogenous mouse hepcidin was replaced by human hepcidin and inflammation was induced using heat-killed Brucella abortus (88). The most effective method was the combination of ESA and anti-hepcidin antibody, which increased haemoglobin by >3 g/dl after 1 week compared with inflamed mice injected with a control antibody. The improvement in haemoglobin resulted from increased serum iron levels and better haemoglobinisation of erythroid precursors, without affecting inflammatory responses in these mice. Pieris pharmaceuticals are conducting a phase 1b placebo-controlled study using a hepcidin-antagonist in patients on dialysis, after demonstrating that the drug was shown to reduce hepcidin levels and increase serum iron and transferrin saturation in 48 healthy male subjects in a single ascending dose study with no significant adverse effects (89).
Vitamin D
Recent studies have shown that vitamin D concentrations are inversely associated with hepcidin levels and positively associated with haemoglobin and iron concentrations (36, 90, 91). Zughaier et al. demonstrated in vitro that vitamin D is associated with reduced production of pro-hepcidin cytokines such as IL-6 and interleukin-1β. In their in vivo pilot study of 38 patients with early stage (2/3) CKD who received high doses of oral vitamin D3 as compared to placebo, the percent change from baseline to 3 months in serum 25-hydroxy cholecalciferol concentrations was inversely associated with the percent change in serum hepcidin levels (92). These findings are relevant as a large majority of patients with CKD are vitamin D deficient, and correction of vitamin D levels, as an adjunct therapy, is attractive, given the inexpensive cost, easy availability, favourable safety profile and potential to reduce dependence on other more expensive therapies.
Future Considerations
Although there is uncertainty surrounding optimal laboratory investigations and haemoglobin targets, our understanding of the mechanisms causing anaemia in CKD has improved over the years. New markers of anaemia are being investigated, and a number of new agents are in evaluation, awaiting completion of phase 2/3 clinical trials. These novel treatments may not only be safer and cheaper but could also reduce our dependence on iron and ESAs by providing the options to manage anaemia using a combination of therapies.
Conflict of interest
The authors declare no conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol. 1999;10(6):1309–16.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol. 1999;10(3):610–19.
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. http://dx.doi.org/10.1126/science.1104742
- Olynyk JK, Trinder D, Ramm GA, Britton RS, Bacon BR. Hereditary hemochromatosis in the post-HFE era. Hepatology. 2008;48(3):991–1001. http://dx.doi.org/10.1002/hep.22507
- Graham RM, Chua AC, Herbison CE, Olynyk JK, Trinder D. Liver iron transport. World J Gastroenterol. 2007;13(35):4725–36. http://dx.doi.org/10.3748/wjg.v13.i35.4725
- Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci. 2007;44(5–6):413–59. http://dx.doi.org/10.1080/10408360701428257
- Beutler E, Hoffbrand AV, Cook JD. Iron deficiency and overload. Hematology Am Soc Hematol Educ Program. 2003:40–61. http://dx.doi.org/10.1182/asheducation-2003.1.40
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331(9):567–73. http://dx.doi.org/10.1056/NEJM199409013310902
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–10. http://dx.doi.org/10.1074/jbc.M008922200
- Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2010;95(3):501–4. http://dx.doi.org/10.3324/haematol.2009.014399
- Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Expression and localization of hepcidin in macrophages: A role in host defense against tuberculosis. J Leukoc Biol. 2007;82(4):934–45. http://dx.doi.org/10.1189/jlb.0407216
- De Domenico I, Zhang TY, Koening CL, Branch RW, London N, LO E, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120(7):2395–405. http://dx.doi.org/10.1172/JCI42011
- Core AB, Canali S, Babitt JL. Hemojuvelin and bone morphogenetic protein (BMP) signaling in iron homeostasis. Front Pharmacol. 2014;5:104. http://dx.doi.org/10.3389/fphar.2014.00104
- Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53(4):1333–41. http://dx.doi.org/10.1002/hep.24178
- Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, et al. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54(1):273–84. http://dx.doi.org/10.1002/hep.24359
- Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–73. http://dx.doi.org/10.1016/S0140-6736(03)12602-5
- Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105(4):1803–6. http://dx.doi.org/10.1182/blood-2004-08-3042
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–44. http://dx.doi.org/10.1172/JCI0215686
- Nemeth E, Rivera S, Gabayan V, Keller V, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6. http://dx.doi.org/10.1172/JCI200420945
- Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, et al. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood. 2012;120(2):431–9. http://dx.doi.org/10.1182/blood-2012-02-411470
- Semenza GL. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985). 2000;88(4):1474–80.
- Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza D, Meijer GA, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol. 2005;206(3):291–304. http://dx.doi.org/10.1002/path.1778
- Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, et al. Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics. 2005;22(3):292–307. http://dx.doi.org/10.1152/physiolgenomics.00217.2004
- Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012;122(12):4635–44. https://dx.doi.org/10.1172/JCI63924
- Piperno A, Galimberti S, Mariani R, Pelucchi S, Ravasi G, Lombardi C, et al. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: Data from the HIGHCARE project. Blood. 2011;117(10):2953–9. http://dx.doi.org/10.1182/blood-2010-08-299859
- Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84. http://dx.doi.org/10.1038/ng.2996
- Kautz L, Nemeth E. Molecular liaisons between erythropoiesis and iron metabolism. Blood. 2014;124(4):479–82. http://dx.doi.org/10.1182/blood-2014-05-516252
- Shoji S, Inaba M, Tomosugi N, Okuno S, Ichii M, Yamakawa T, et al. Greater potency of darbepoetin-alpha than erythropoietin in suppression of serum hepcidin-25 and utilization of iron for erythropoiesis in hemodialysis patients. Eur J Haematol. 2013;90(3):237–44. http://dx.doi.org/10.1111/ejh.12067
- Onuma S, Honda H, Kobayashi Y, Yamamoto T, Michihata T, Shibagaki K, et al. Effects of long-term erythropoiesis-stimulating agents on iron metabolism in patients on hemodialysis. Ther Apher Dial. 2015;19(6):582–9. http://dx.doi.org/10.1111/1744-9987.12322
- Honda H, Kobayashi Y, Onuma S, Shibagaki K, Yuza T, Hirao K, et al. Associations among erythroferrone and biomarkers of erythropoiesis and iron metabolism, and treatment with long-term erythropoiesis-stimulating agents in patients on hemodialysis. PLoS One. 2016;11(3):e0151601. http://dx.doi.org/10.1371/journal.pone.0151601
- McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: A population-based study. J Am Soc Nephrol. 2002;13(7):1928–36. http://dx.doi.org/10.1097/01.ASN.0000018409.45834.FA
- Astor BC, Arnett DK, Brown A, Coresh J. Association of kidney function and hemoglobin with left ventricular morphology among African Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2004;43(5):836–45. http://dx.doi.org/10.1053/j.ajkd.2003.12.047
- Panwar B, Gutierrez OM. Disorders of iron metabolism and anemia in chronic kidney disease. Semin Nephrol. 2016;36(4):252–61. http://dx.doi.org/10.1016/j.semnephrol.2016.05.002
- Young B, Zaritsky J. Hepcidin for clinicians. Clin J Am Soc Nephrol. 2009;4(8):1384–7. http://dx.doi.org/10.2215/CJN.02190309
- Kuragano T, Shimonaka Y, Kida A, Furuta M, Nanami M, Otaki Y, et al. Determinants of hepcidin in patients on maintenance hemodialysis: Role of inflammation. Am J Nephrol. 2010;31(6):534–40. http://dx.doi.org/10.1159/000312381
- Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, et al. Hepcidin – A potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1051–6. http://dx.doi.org/10.2215/CJN.05931108
- Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75(9):976–81. http://dx.doi.org/10.1038/ki.2009.21
- Weiss G, Theurl I, Eder S, Koppelstaetter C, Kurz K, Sonnweber T, et al. Serum hepcidin concentration in chronic haemodialysis patients: Associations and effects of dialysis, iron and erythropoietin therapy. Eur J Clin Invest. 2009;39(10):883–90. http://dx.doi.org/10.1111/j.1365-2362.2009.02182.x
- Milward EA, Trinder D, Wilcox CE, Britton RS, Ramm GA, Olynyk JK. Is HFE involved in increased hepcidin expression and hypoferremia in inflammation and anemia of chronic disease? Hepatology. 2005;41(4):936–8. http://dx.doi.org/10.1002/hep.20652
- Eleftheriadis T, Liakopoulos V, Antoniadi G, Kartsios C, Stefanidis I. The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin Dial. 2009;22(1):70–7. http://dx.doi.org/10.1111/j.1525-139X.2008.00532.x
- Pecoits-Filho R, Lindholm B, Axelsson J, Stenvinkel P. Update on interleukin-6 and its role in chronic renal failure. Nephrol Dial Transplant. 2003;18(6):1042–5. http://dx.doi.org/10.1093/ndt/gfg111
- Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–7. http://dx.doi.org/10.1182/blood-2008-02-139915
- Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: Implications for the anemia of CKD. Am J Kidney Dis. 2010;55(4):726–41. http://dx.doi.org/10.1053/j.ajkd.2009.12.030
- Vos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ. Red blood cell survival in long-term dialysis patients. Am J Kidney Dis. 2011;58(4):591–8. http://dx.doi.org/10.1053/j.ajkd.2011.03.031
- Eschbach JW Jr., Funk D, Adamson J, Kuhn I, Scribner BH, Finch CA. Erythropoiesis in patients with renal failure undergoing chronic dialysis. N Engl J Med. 1967;276(12):653–8. http://dx.doi.org/10.1056/NEJM196703232761202
- Alon DB, Chaimovitz C, Dvilansky A, Lugassy G, Douvdevani A, Shany S, et al. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp Hematol. 2002;30(5):403–9. http://dx.doi.org/10.1016/S0301-472X(02)00789-0
- Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K, et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014;25(3):564–72. http://dx.doi.org/10.1681/ASN.2013040355
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet. 2006;367(9505):133–43. http://dx.doi.org/10.1016/S0140-6736(06)67962-2
- Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195(12):1745–53. http://dx.doi.org/10.1086/518040
- McDermid JM, Jaye A, Schim van der Loeff MF, Todd J, Bates C, Austin S, et al. Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immune Defic Syndr. 2007;46(4):498–507. http://dx.doi.org/10.1097/QAI.0b013e31815b2d4b
- Litton E, Baker S, Erber W, French C, Ferrier J, Hawkins D, et al. The IRONMAN trial: A protocol for a multicentre randomised placebo-controlled trial of intravenous iron in intensive care unit patients with anaemia. Crit Care Resusc. 2014;16(4):285–90.
- Barany P, Eriksson LC, Hultcrantz R, Pettersson E, Bergstrom J. Serum ferritin and tissue iron in anemic dialysis patients. Miner Electrolyte Metab. 1997;23(3–6):273–6.
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: Molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–97. http://dx.doi.org/10.1016/S0092-8674(04)00343-5
- Saeed O, Otsuka F, Polavarapu R, Karmali V, Weiss D, Davis T, et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(2):299–307. http://dx.doi.org/10.1161/ATVBAHA.111.240101
- Wilson JG. Iron and glucose homeostasis: New lessons from hereditary haemochromatosis. Diabetologia. 2006;49(7):1459–61. http://dx.doi.org/10.1007/s00125-006-0289-1
- Drueke T, Witko-Sarsat V, Massy Z, Descamps-Latscha Z, Guerin AP, Marchais SJ, et al. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002;106(17):2212–17. http://dx.doi.org/10.1161/01.CIR.0000035250.66458.67
- van der Weerd NC, Grooteman MP, Bots ML, van den Dorpel MA, den Hoedt CH, Mazairac AH, et al. Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol Dial Transplant. 2013;28(12):3062–71. http://dx.doi.org/10.1093/ndt/gfs488
- Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: Evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol. 2004;24(5):949–54. http://dx.doi.org/10.1161/01.ATV.0000124892.90999.cb
- Finn AV, Nakano M, Polavarapu R, Karmal V, Saeed O, Zhao X, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59(2):166–77. http://dx.doi.org/10.1016/j.jacc.2011.10.852
- Del Vecchio L, Longhi S, Locatelli F. Safety concerns about intravenous iron therapy in patients with chronic kidney disease. Clin Kidney J. 2016;9(2):260–7. http://dx.doi.org/10.1093/ckj/sfv142
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–90. http://dx.doi.org/10.1056/NEJM199808273390903
- Besarab A, Goodkin DA, Nissenson AR, Normal Hematocrit Cardiac Trial Authors. The normal hematocrit study – Follow-up. N Engl J Med. 2008;358(4):433–4. http://dx.doi.org/10.1056/NEJMc076523
- Levin NW, Lazarus JM, Nissenson AR. National cooperative rHu erythropoietin study in patients with chronic renal failure – An interim report. The national cooperative rHu erythropoietin study group. Am J Kidney Dis. 1993;22(2 Suppl 1):3–12. http://dx.doi.org/10.1016/0272-6386(93)70176-Y
- Parfrey PS, Lauve M, Latremouille-Viau D, Lefebvre P. Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: A meta-analysis. Clin J Am Soc Nephrol. 2009;4(4):755–62. http://dx.doi.org/10.2215/CJN.02730608
- Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–84. http://dx.doi.org/10.1056/NEJMoa062276
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–98. http://dx.doi.org/10.1056/NEJMoa065485
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–32. http://dx.doi.org/10.1056/NEJMoa0907845
- Winkelmayer WC. Against TREATing all patients alike: Lessons from an FDA advisory committee meeting. J Am Soc Nephrol. 2011;22(1):1–2. http://dx.doi.org/10.1681/ASN.2010111133
- Liu Y, Xu Y, Thilo F, Friis UG, Jensen BL, Scholze A, et al. Erythropoietin increases expression and function of transient receptor potential canonical 5 channels. Hypertension. 2011;58(2):317–24. http://dx.doi.org/10.1161/HYPERTENSIONAHA.111.173690
- Janmaat ML, Heerkens JL, de Bruin AM, Klous A, de Waard V, de Vries CJ. Erythropoietin accelerates smooth muscle cell-rich vascular lesion formation in mice through endothelial cell activation involving enhanced PDGF-BB release. Blood. 2010;115(7):1453–60. http://dx.doi.org/10.1182/blood-2009-07-230870
- Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin Cancer Res. 2011;17(20):6373–80. http://dx.doi.org/10.1158/1078-0432.CCR-10-2577
- Hung SC, Lin YP, Tarng DC. Erythropoiesis-stimulating agents in chronic kidney disease: What have we learned in 25 years? J Formos Med Assoc. 2014;113(1):3–10. http://dx.doi.org/10.1016/j.jfma.2013.09.004
- Freitas JP, Filipe PM. Pentoxifylline. A hydroxyl radical scavenger. Biol Trace Elem Res. 1995;47(1–3):307–11. http://dx.doi.org/10.1007/BF02790131
- Bienvenu J, Doche C, Gutowski MC, Lenoble M, Lepape A, Perdrix JP. Production of proinflammatory cytokines and cytokines involved in the TH1/TH2 balance is modulated by pentoxifylline. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S80–4. http://dx.doi.org/10.1097/00005344-199500252-00017
- Benbernou N, Esnault S, Potron G, Guenounou M. Regulatory effects of pentoxifylline on T-helper cell-derived cytokine production in human blood cells. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S75–9. http://dx.doi.org/10.1097/00005344-199500252-00016
- Johnson DW, Pascoe EM, Badve SV, Dalziel K, Cass A, Clarke P, et al. A randomized, placebo-controlled trial of pentoxifylline on erythropoiesis-stimulating agent hyporesponsiveness in anemic patients with CKD: The Handling Erythropoietin Resistance with Oxpentifylline (HERO) trial. Am J Kidney Dis. 2015;65(1):49–57. http://dx.doi.org/10.1053/j.ajkd.2014.06.020
- Gummer J, Trengove R, Pascoe EM, Badve SV, Cass A, Clarke P, et al. Association between serum hepcidin-25 and primary resistance to erythropoiesis stimulating agents in chronic kidney disease: A secondary analysis of the HERO trial. Nephrology (Carlton). 2016. http://dx.doi.org/10.1111/nep.12815 [Epub ahead of print].
- Ferrari P, Mallon D, Trinder D, Olynyk JK. Pentoxifylline improves haemoglobin and interleukin-6 levels in chronic kidney disease. Nephrology (Carlton). 2010;15(3):344–9. http://dx.doi.org/10.1111/j.1440-1797.2009.01203.x
- Bolignano D, D’Arrigo G, Pisano A, Coppolino G. Pentoxifylline for anemia in chronic kidney disease: A systematic review and meta-analysis. PLoS One. 2015;10(8):e0134104. http://dx.doi.org/10.1371/journal.pone.0134104
- Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, et al. Roxadustat (FG-4592): Correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27(4):1225–33. http://dx.doi.org/10.1681/ASN.2015030241
- Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90(5):1115–22. http://dx.doi.org/10.1016/j.kint.2016.07.019
- Flamme I, Oehme F, Ellinghaus P, Jeske M, Keldenich J, Thuss U. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One. 2014;9(11):e111838. http://dx.doi.org/10.1371/journal.pone.0111838
- Holdstock L, Meadowcroft AM, Maier R, Johnson BM, Jones D, Rastogi A, et al. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27(4):1234–44. http://dx.doi.org/10.1681/ASN.2014111139
- Maxwell PH, Eckardt KU. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol. 2016;12(3):157–68. http://dx.doi.org/10.1038/nrneph.2015.193
- Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer. 2011;2(12):1117–33. http://dx.doi.org/10.1177/1947601911423654
- Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, et al. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol. 2016;36(10):1584–94. http://dx.doi.org/10.1128/MCB.01055-15
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. http://dx.doi.org/10.1146/annurev-pathol-012513-104720
- Cooke KS, Hinkle B, Salimi-Moosavi H, Foltz I, King C, Rathanaswami P, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122(17):3054–61. http://dx.doi.org/10.1182/blood-2013-06-505792
- Badve SV, Palmer SC, Strippoli GF, Roberts MA, Teixeira- Pinto A, Boudville N, et al. The validity of left ventricular mass as a surrogate end point for all-cause and cardiovascular mortality outcomes in people with CKD: A systematic review and meta-analysis. Am J Kidney Dis. 2016;68(4):554–63. http://dx.doi.org/10.1053/j.ajkd.2016.03.418
- Carvalho C, Isakova T, Collerone G, Olbina G, Wolf M, Westerman M, et al. Hepcidin and disordered mineral metabolism in chronic kidney disease. Clin Nephrol. 2011;76(2):90–8. http://dx.doi.org/10.5414/CN107018
- Icardi A, Paoletti E, De Nicola L, Mazzaferro S, Russo R, Cozzolino M. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: The potential role of inflammation. Nephrol Dial Transplant. 2013;28(7):1672–9. http://dx.doi.org/10.1093/ndt/gft021
- Zughaier SM, Alvarez JA, Sloan JH, Konrad RJ, Tangpricha V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J Clin Transl Endocrinol. 2014;1(1):19–25. http://dx.doi.org/10.1016/j.jcte.2014.01.003